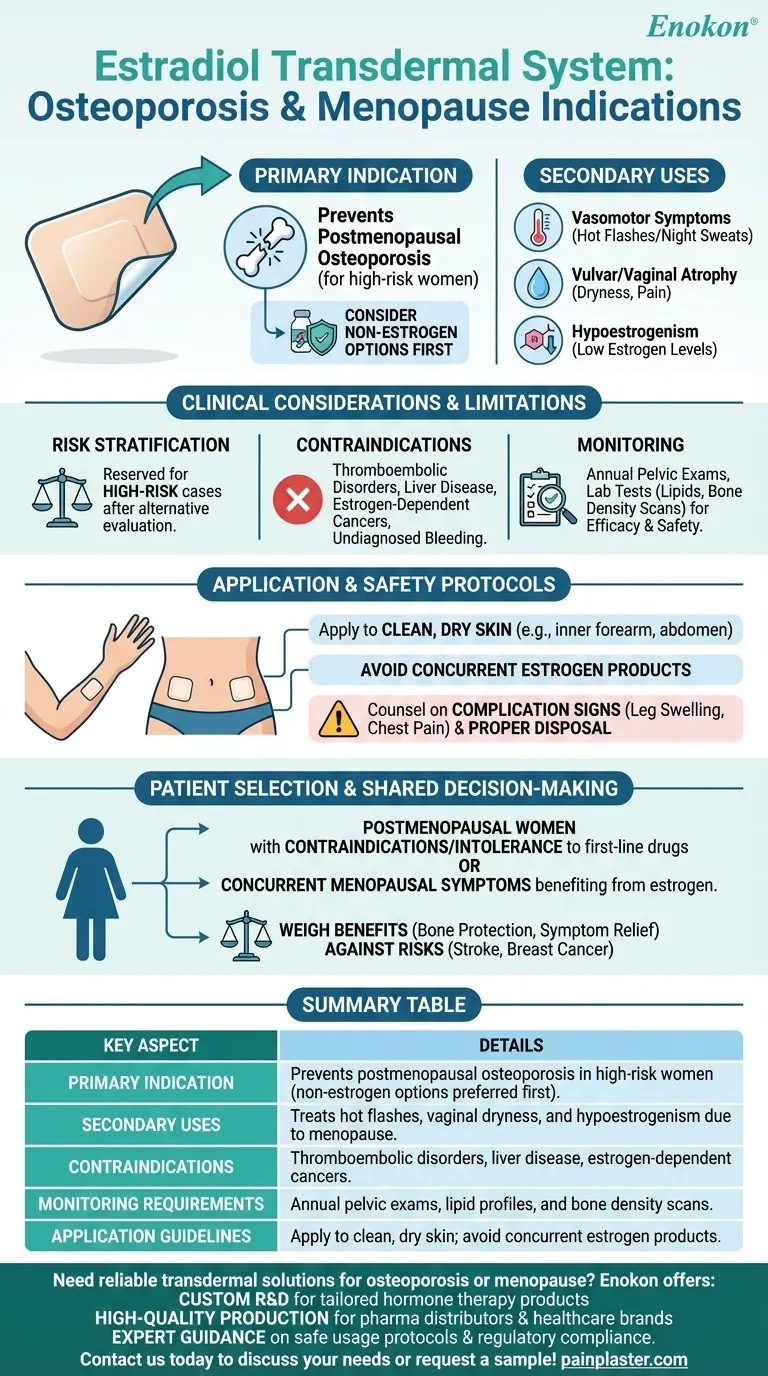
The estradiol transdermal system, specifically the Estradiol Transdermal Patch, is primarily indicated for preventing postmenopausal osteoporosis in women at significant risk, with the caveat that non-estrogen treatments should be considered first. It is also approved for managing menopausal symptoms like hot flashes, vaginal dryness, and hypoestrogenism. However, its use for osteoporosis prevention requires careful evaluation of individual risk factors and contraindications, including thromboembolic disorders, liver disease, and estrogen-dependent cancers. Regular monitoring, including pelvic exams and lab tests, is essential to ensure safe usage.
Key Points Explained:
-
Primary Indication for Osteoporosis Prevention
- The estradiol transdermal system is FDA-approved to prevent postmenopausal osteoporosis, but only for women at significant risk (e.g., those with low bone density or fractures).
- Non-estrogen therapies (e.g., bisphosphonates) are preferred first-line options due to safety concerns with long-term estrogen use.
-
Secondary Uses in Menopause Management
- Beyond osteoporosis, the patch treats:
- Moderate-to-severe vasomotor symptoms (hot flashes/night sweats).
- Vulvar/vaginal atrophy (dryness, pain during intercourse).
- Hypoestrogenism (low estrogen levels due to surgical/natural menopause).
- Beyond osteoporosis, the patch treats:
-
Clinical Considerations and Limitations
- Risk stratification: Estrogen therapy is reserved for high-risk osteoporosis cases after evaluating alternatives.
- Contraindications: Includes history of thromboembolism, liver disease, estrogen-sensitive cancers, or undiagnosed vaginal bleeding.
- Monitoring: Requires annual pelvic exams and lab tests (e.g., lipid profiles, bone density scans) to assess efficacy and safety.
-
Application and Safety Protocols
- Applied to clean, dry skin (e.g., inner forearm or abdomen).
- Avoid concurrent use with other estrogen products to prevent overdose.
- Patients must be counseled on signs of complications (e.g., leg swelling, chest pain) and proper disposal to prevent accidental exposure to children/pets.
-
Patient Selection and Shared Decision-Making
- Ideal candidates are postmenopausal women with:
- Contraindications/intolerance to first-line osteoporosis drugs.
- Concurrent menopausal symptoms benefiting from estrogen.
- Shared decision-making should weigh benefits (bone protection, symptom relief) against risks (stroke, breast cancer).
- Ideal candidates are postmenopausal women with:
This targeted approach ensures the patch is used safely and effectively, aligning with guidelines that prioritize individualized care in postmenopausal health.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Indication | Prevents postmenopausal osteoporosis in high-risk women (non-estrogen options preferred first). |
| Secondary Uses | Treats hot flashes, vaginal dryness, and hypoestrogenism due to menopause. |
| Contraindications | Thromboembolic disorders, liver disease, estrogen-dependent cancers. |
| Monitoring Requirements | Annual pelvic exams, lipid profiles, and bone density scans. |
| Application Guidelines | Apply to clean, dry skin; avoid concurrent estrogen products. |
Need reliable transdermal solutions for osteoporosis or menopause?
As a bulk manufacturer of FDA-compliant estradiol patches, Enokon offers:
- Custom R&D for tailored hormone therapy products.
- High-quality production for pharma distributors and healthcare brands.
- Expert guidance on safe usage protocols and regulatory compliance.
Contact us today to discuss your needs or request a sample!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints














