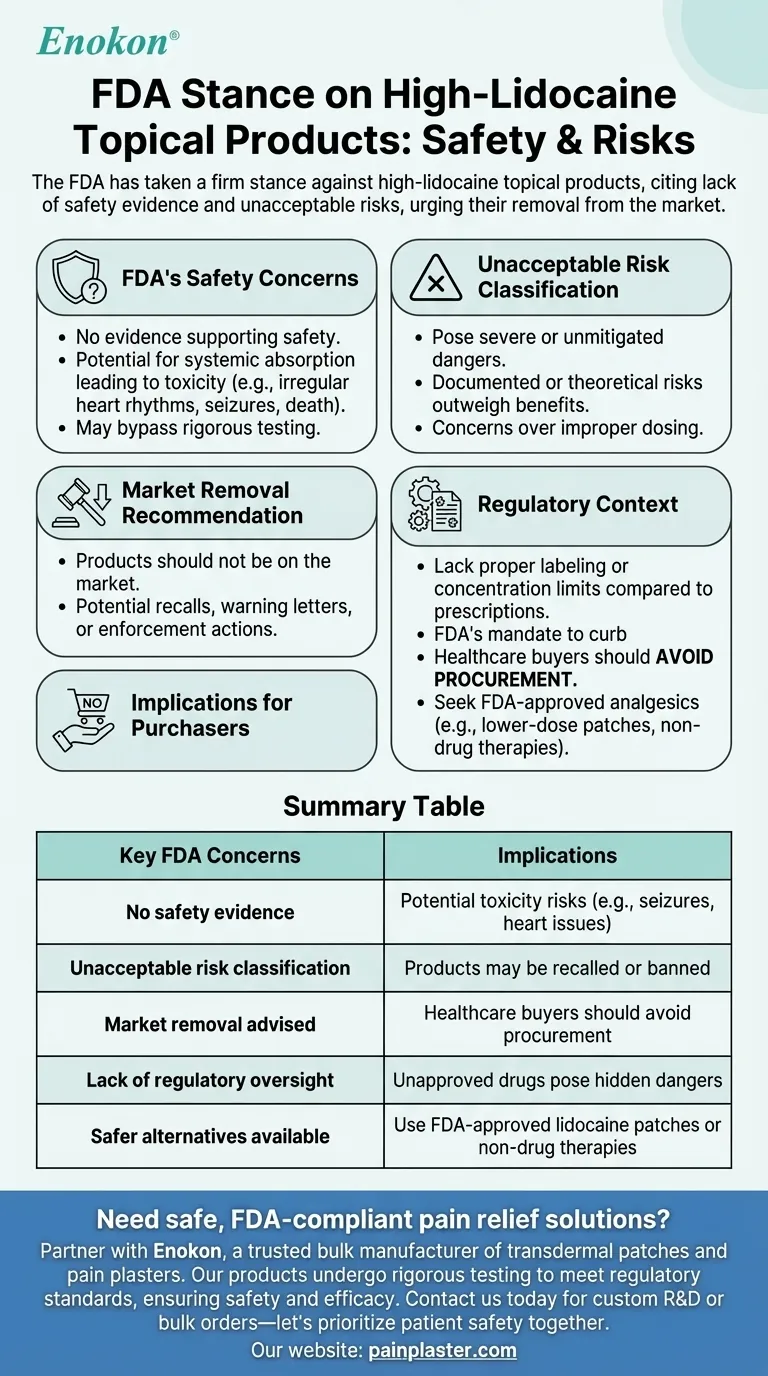
The FDA has taken a firm stance against high-lidocaine topical products, citing a lack of evidence for their safety and deeming them to present unacceptable risks to consumers. The agency explicitly states these products should not be available on the market due to potential health hazards. This position reflects concerns over unverified safety claims and the potential for misuse or adverse effects, emphasizing the need for stricter regulatory oversight to protect public health.

Key Points Explained:
-
FDA's Safety Concerns
- The FDA has no evidence supporting the safety of high-lidocaine topical products.
- This lack of data raises red flags about potential risks, such as systemic absorption leading to toxicity (e.g., irregular heart rhythms, seizures, or even death in extreme cases).
- The agency’s stance implies these products may bypass rigorous testing required for approved drugs, increasing uncertainty about their effects.
-
Unacceptable Risk Classification
- The FDA categorizes these products as posing unacceptable risks, a rare designation reserved for items with severe or unmitigated dangers.
- This suggests documented adverse events or theoretical risks (e.g., improper dosing due to lack of standardization) outweigh any perceived benefits.
-
Market Removal Recommendation
- The assertion that these products should not be on the market underscores the FDA’s proactive approach to consumer protection.
- This may prompt recalls, warning letters to manufacturers, or enforcement actions against distributors.
-
Regulatory Context
- Unlike prescription lidocaine (which has dosage controls and clinical oversight), OTC high-lidocaine products may lack proper labeling or concentration limits.
- The FDA’s position aligns with its mandate to curb unapproved drugs masquerading as topical remedies.
-
Implications for Purchasers
- Healthcare buyers should avoid procurement of such products until further evidence or reformulation addresses FDA concerns.
- Alternative, FDA-approved analgesics (e.g., lower-dose lidocaine patches or non-drug therapies) may be safer substitutes.
The FDA’s warning serves as a critical reminder of the hidden dangers in seemingly benign OTC products, urging both consumers and professionals to prioritize verified safety data over anecdotal claims.
Summary Table:
| Key FDA Concerns | Implications |
|---|---|
| No safety evidence | Potential toxicity risks (e.g., seizures, heart issues) |
| Unacceptable risk classification | Products may be recalled or banned |
| Market removal advised | Healthcare buyers should avoid procurement |
| Lack of regulatory oversight | Unapproved drugs pose hidden dangers |
| Safer alternatives available | Use FDA-approved lidocaine patches or non-drug therapies |
Need safe, FDA-compliant pain relief solutions? Partner with Enokon, a trusted bulk manufacturer of transdermal patches and pain plasters for healthcare distributors and brands. Our products undergo rigorous testing to meet regulatory standards, ensuring safety and efficacy. Contact us today for custom R&D or bulk orders—let’s prioritize patient safety together.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- For what condition are lidocaine patches approved in the United Kingdom? A Guide to Postherpetic Neuralgia Treatment
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
















