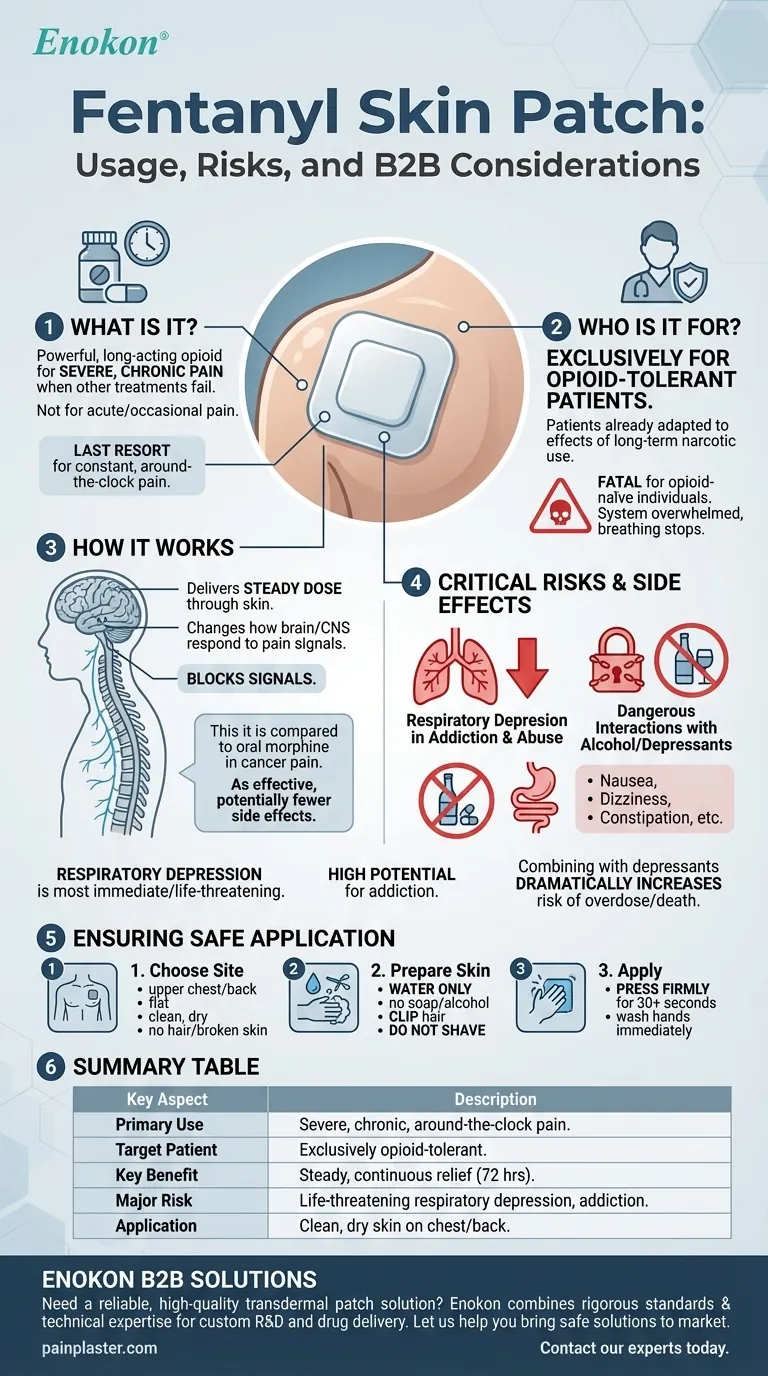
In short, the fentanyl skin patch is a powerful opioid medication used to manage severe, chronic pain when other treatments are no longer effective. It is a long-acting solution prescribed exclusively for patients who are already tolerant to other narcotic pain medications due to its potency and significant risks.
The fentanyl patch is a tool of last resort for managing persistent, around-the-clock pain. Its effectiveness is balanced by a high risk of life-threatening side effects, including addiction and respiratory failure, demanding strict medical supervision.
The Role of the Fentanyl Patch in Pain Management
A Tool for Severe, Chronic Pain
The fentanyl patch is designed to deliver a steady, continuous dose of medication through the skin over a period of several days. This makes it suitable for managing constant pain, such as that associated with advanced cancer.
It is not intended for sudden (acute) pain, post-surgical pain, or pain that is only occasional. The slow-release mechanism cannot provide the rapid relief needed for these situations.
Defining "Opioid-Tolerant"
This medication is prescribed only for patients who are opioid-tolerant. This means their body has already adapted to the effects of narcotic pain medications from previous, long-term use.
Prescribing fentanyl to someone who is not opioid-tolerant can be fatal, as their system would be overwhelmed by the drug's potent effects on breathing.
How It Works
The fentanyl in the patch works by changing how the brain and central nervous system respond to pain signals. By blocking these signals, it provides a powerful and consistent level of pain relief that may not be achievable with other medications.
Studies have shown it to be as effective as oral morphine for cancer pain, often with fewer side effects like nausea and vomiting.
Critical Risks and Serious Side Effects
The Danger of Respiratory Depression
The most immediate and life-threatening risk of fentanyl is respiratory depression, which is the dangerous slowing or complete stopping of breathing. This risk is highest when first starting the patch or after a dose increase.
High Potential for Addiction and Abuse
Fentanyl carries a very high risk for abuse and addiction, which can easily lead to overdose and death. It is a controlled substance for this reason, and its use must be closely monitored by a healthcare professional.
Dangerous Drug Interactions
Combining the fentanyl patch with alcohol or other central nervous system depressants (like sedatives, tranquilizers, or other opioids) is extremely dangerous. This combination dramatically increases the risk of severe drowsiness, respiratory depression, coma, and death.
Common Side Effects
Patients may experience side effects such as nausea, vomiting, constipation, lightheadedness, dizziness, or drowsiness. Mild skin irritation, redness, or itching at the application site can also occur. For some, these effects may lessen over time.
Ensuring Safe and Proper Application
Choosing the Application Site
The patch must be applied to a flat, clean, and dry area of skin, typically on the upper chest or back. The upper back is often recommended for children or individuals with cognitive impairment to prevent them from removing it.
Avoid applying the patch to skin that is oily, broken, irritated, cut, or burned.
Preparing the Skin
The chosen area should be cleaned with water only. Do not use soaps, oils, lotions, or alcohol, as they can affect how the medication is absorbed.
If the area has hair, it should be clipped short with scissors. Do not shave the area, as this can irritate the skin and alter medication absorption.
The Application Process
Press the patch firmly onto the prepared skin with the palm of your hand for at least 30 seconds to ensure it is sealed correctly. After application, wash your hands immediately with water.
Understanding the Trade-offs
Benefit: Powerful, Consistent Relief
For the right patient, the fentanyl patch provides a level of powerful, around-the-clock pain relief that is difficult to achieve with other medications. Its steady-state delivery can significantly improve quality of life when chronic pain is debilitating.
Limitation: A Narrow Margin of Safety
The difference between a therapeutic dose and a life-threatening one is very small. Accidental exposure, incorrect application, or using a damaged patch can lead to a fatal overdose. Heat sources (like heating pads or hot tubs) applied over the patch can also cause a dangerous increase in medication release.
Risk: Not for the Opioid-Naïve
This cannot be overstated: the fentanyl patch is only for patients whose bodies are already accustomed to strong opioids. For anyone else, a standard dose can be fatal.
Making the Right Choice for Your Goal
- If your goal is to manage severe, constant pain that no longer responds to other treatments: The fentanyl patch may be a viable option, but only under the strict guidance of a physician who has determined you are opioid-tolerant.
- If your goal is to treat sudden, intermittent, or post-surgical pain: The fentanyl patch is absolutely not the right tool and would be dangerously inappropriate for your needs.
- If you are a caregiver for someone using the patch: Your primary focus must be on safe application, preventing accidental exposure to others, and immediately recognizing the signs of an overdose, such as slowed breathing or unresponsiveness.
Ultimately, the fentanyl patch is a specialized medical tool that requires a deep understanding of its profound benefits and life-threatening risks.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Use | Management of severe, chronic, around-the-clock pain (e.g., cancer pain). |
| Target Patient | Exclusively for patients who are already opioid-tolerant. |
| Key Benefit | Provides steady, continuous pain relief over 72 hours. |
| Major Risk | Life-threatening respiratory depression; high potential for addiction. |
| Application | Applied to clean, dry, non-irritated skin on the upper chest or back. |
Need a reliable, high-quality transdermal patch solution for your healthcare brand or distribution network?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we combine rigorous manufacturing standards with deep technical expertise. We partner with pharmaceutical distributors and healthcare brands to deliver custom R&D and development for specialized drug delivery systems.
Let us help you bring safe and effective transdermal solutions to market. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- When should the pain relief patch not be used? Key Safety Rules to Avoid Risks
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing


















