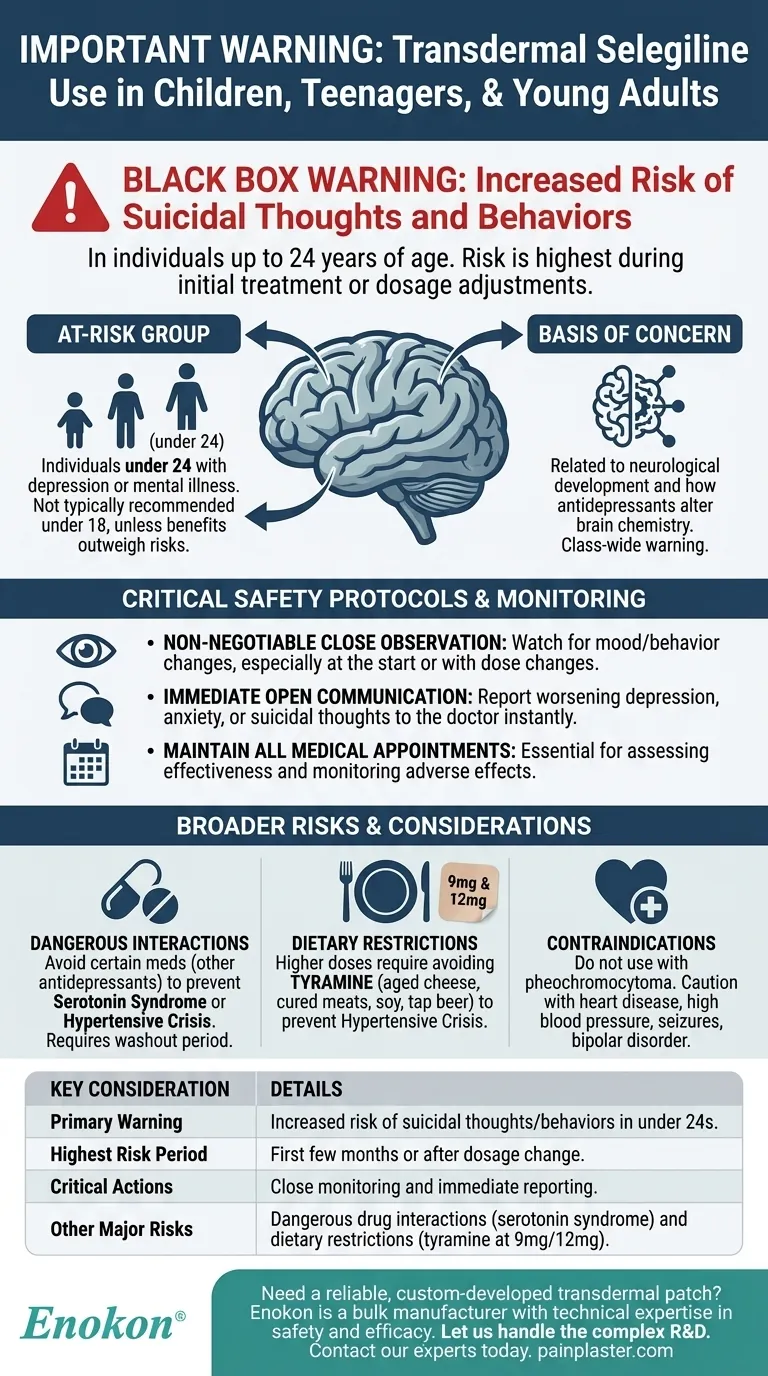
The most important warning regarding transdermal selegiline is that like other antidepressants, it can increase the risk of suicidal thoughts and behaviors in children, teenagers, and young adults up to 24 years of age. This risk was identified during clinical studies and requires vigilant monitoring, particularly when treatment begins or the dose is adjusted.
While transdermal selegiline can be an effective treatment, its use in younger populations is approached with significant caution. The core issue is not that the drug causes suicidality, but that it can potentially heighten these thoughts in individuals who are already vulnerable due to their underlying mental illness.
Unpacking the Warning: Understanding Suicidal Ideation
The warning about increased suicidal thoughts is what is known as a "black box warning"—the most serious type issued by the FDA. It is based on observations from a small but statistically significant number of individuals in clinical trials.
Who Is Most at Risk?
The identified at-risk group includes children, teenagers, and young adults up to the age of 24. Individuals in this age bracket being treated for depression or other mental illnesses may be more susceptible to this effect.
The Basis of the Concern
Experts are not certain of the exact mechanism behind this risk. It is a class-wide warning for many antidepressants, suggesting it's related to the complex way these medications alter brain chemistry during a period of significant neurological development.
The Physician's Calculation
Ordinarily, transdermal selegiline is not recommended for individuals under 18. However, a specialist may determine that the potential benefits of treating a severe condition outweigh the known risks, making it the most appropriate choice in a specific case. This decision is made only after careful evaluation.
Critical Safety Protocols and Monitoring
Because of this risk, a strict protocol of observation and communication is essential for any young person prescribed transdermal selegiline.
Why Close Observation is Non-Negotiable
The highest risk for developing these thoughts is typically at the beginning of treatment or following any change in dosage. Caregivers and patients must watch for any unusual changes in mood, behavior, or the emergence of suicidal thoughts.
The Importance of Open Communication
Any new or sudden changes in mood, such as worsening depression, anxiety, agitation, panic attacks, or irritability, must be reported to the prescribing doctor immediately. Open dialogue between the patient, family, and healthcare provider is the primary tool for managing this risk.
Maintaining All Medical Appointments
Regular follow-up appointments are not optional. They are a critical component of the treatment plan, allowing the doctor to assess the medication's effectiveness and monitor for any adverse effects.
Understanding the Broader Risks and Trade-offs
Beyond the primary warning for young adults, transdermal selegiline carries other significant safety considerations that require careful management.
Dangerous Drug Interactions
Combining selegiline with certain other medications, especially other antidepressants, can be extremely dangerous. It can lead to serotonin syndrome (a life-threatening condition caused by excess serotonin) or a hypertensive crisis (a severe, rapid increase in blood pressure). A "washout period" of one to five weeks is often required when switching from another antidepressant.
Dietary Restrictions with Higher Doses
At the lowest dose (6 mg patch), dietary restrictions are not typically needed. However, at higher doses (9 mg and 12 mg patches), patients must avoid foods high in tyramine. These include aged cheeses, cured meats, fermented foods like sauerkraut, soy products, and tap beer. Ingesting tyramine while on higher doses can trigger a hypertensive crisis.
Contraindications and Pre-existing Conditions
This medication should not be used by individuals with a pheochromocytoma (an adrenal gland tumor). It must be used with extreme caution in patients with a history of heart disease, high blood pressure, seizures, or bipolar disorder.
Making the Right Choice for Your Goal
Navigating the use of this medication requires a clear understanding of its risks and a commitment to strict safety protocols.
- If your primary focus is the safety of a young person (under 24): Prioritize immediate and open communication with the doctor about any change in mood or thoughts of self-harm.
- If you are managing multiple medications: Ensure your doctor and pharmacist have a complete, updated list of all drugs and supplements to prevent life-threatening interactions.
- If you are using a higher dose (9 mg or 12 mg): You must strictly adhere to the tyramine-restricted diet to avoid a dangerous spike in blood pressure.
Understanding these risks is the first and most critical step toward using this medication safely and effectively.
Summary Table:
| Key Consideration | Details |
|---|---|
| Primary Warning | Increased risk of suicidal thoughts and behaviors in children, teenagers, and young adults (up to age 24). |
| Highest Risk Period | During the first few months of treatment or after a dosage change. |
| Critical Actions | Close monitoring by family/doctors and immediate reporting of any mood or behavior changes. |
| Other Major Risks | Dangerous drug interactions (serotonin syndrome) and dietary restrictions (tyramine) at higher doses (9mg/12mg). |
Need a reliable, custom-developed transdermal patch?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your product is developed with the highest standards of safety and efficacy in mind.
Let us handle the complex R&D so you can focus on patient care.
Contact our experts today to discuss your custom transdermal patch development needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism

















