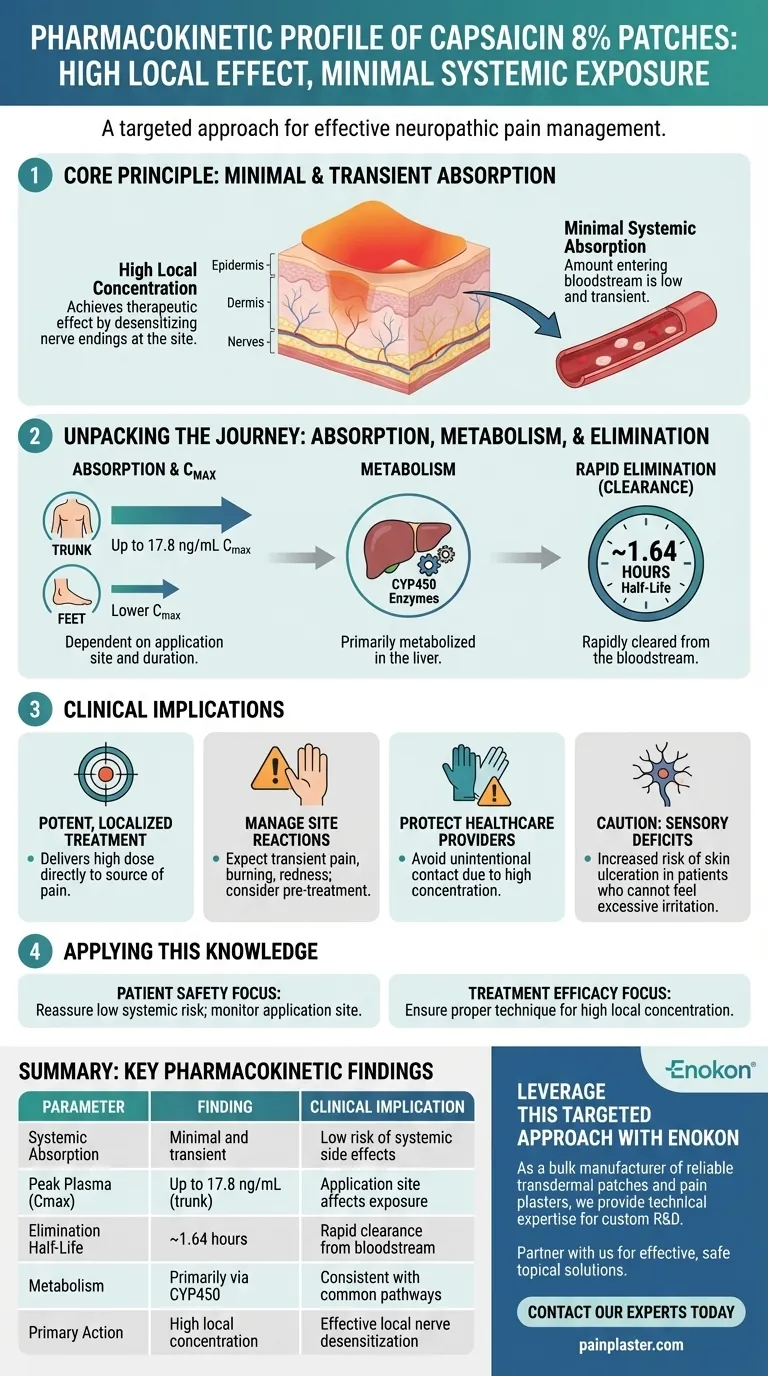
In short, the capsaicin 8% patch has a pharmacokinetic profile defined by minimal and transient systemic absorption. Its therapeutic effect is achieved through high local drug concentration at the application site, while the amount that enters the bloodstream is low, depends on the application area, and is cleared from the body quickly.
The core principle of the capsaicin 8% patch is to maximize local nerve desensitization while minimizing systemic exposure. Its pharmacokinetic profile confirms this design, showing that the drug acts locally and is rapidly eliminated if any enters the bloodstream, which underpins both its efficacy and safety.

Unpacking the Pharmacokinetic Journey
Understanding how the capsaicin 8% patch moves through the body is key to using it safely and effectively. The process is designed to keep the drug's powerful effects contained to the target area.
Absorption: A Highly Localized Effect
Systemic absorption of capsaicin from the 8% patch is minimal. The amount that enters the bloodstream is dependent on both the duration of application and the concentration of the patch.
This design is intentional, allowing the drug to reach very high concentrations in the skin to desensitize nerve endings without causing significant effects elsewhere in the body.
Peak Plasma Concentration (Cmax)
The location of the patch significantly impacts peak plasma levels. Application to the trunk can result in a peak plasma level of 17.8 ng/mL.
In contrast, application to the feet yields lower concentrations. This is likely due to differences in skin thickness and blood flow between these areas, with the trunk's thinner skin and greater vascularity allowing for slightly more absorption.
Metabolism and Elimination (Clearance)
Once in the bloodstream, capsaicin has a very short elimination half-life of approximately 1.64 hours. This means the body clears half of the absorbed drug in under two hours.
It is metabolized primarily in the liver by the cytochrome P450 enzyme system, a common pathway for many drugs. This rapid clearance ensures that the minimal amount of absorbed drug does not linger in the system.
Clinical Implications of This Profile
The pharmacokinetic data directly informs how the patch should be handled in a clinical setting and highlights critical safety considerations.
The Rationale for Potent, Localized Treatment
Because systemic exposure is so low, the patch can deliver a high dose of capsaicin directly to the source of neuropathic pain. This high local concentration is what effectively "defunctionalizes" pain-sensing nerve fibers.
Managing Application-Site Reactions
The potent local effect invariably causes transient pain, burning, and redness. This is a direct consequence of the drug's mechanism. Pre-treatment with analgesics is often recommended to manage this predictable, on-target effect.
Protecting the Healthcare Provider
The high concentration of capsaicin in the patch means that unintentional contact must be avoided by healthcare staff. The pharmacokinetics show the drug is potent at the site of contact, reinforcing the need for careful handling by trained personnel.
Caution in Patients with Sensory Deficits
Patients with impaired sensory function, such as those with diabetic neuropathy, require extreme caution. They may not be able to properly feel excessive irritation or chemical burns, increasing the risk of skin ulceration.
Applying This Knowledge in Practice
Understanding these pharmacokinetics is essential for translating the treatment from theory to safe, effective clinical application.
- If your primary focus is patient safety: Reassure them that systemic side effects are unlikely due to minimal absorption and rapid clearance, but emphasize the critical need to monitor the application site for skin reactions, especially in at-risk populations.
- If your primary focus is treatment efficacy: This profile confirms the drug acts locally, meaning proper application technique and site preparation are paramount to ensuring the high concentration reaches the target nerves.
By understanding this profile, you can confidently leverage a potent topical therapy for neuropathic pain precisely as it was designed.
Summary Table:
| Pharmacokinetic Parameter | Key Finding | Clinical Implication |
|---|---|---|
| Systemic Absorption | Minimal and transient | Low risk of systemic side effects |
| Peak Plasma (Cmax) | Up to 17.8 ng/mL (trunk application) | Application site affects exposure |
| Elimination Half-Life | ~1.64 hours | Rapid clearance from bloodstream |
| Metabolism | Primarily via CYP450 enzymes in liver | Consistent with common drug pathways |
| Primary Action | High local concentration at site | Effective local nerve desensitization |
Leverage this targeted drug delivery approach for your pain management products. As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide the technical expertise for custom R&D and development. Partner with us to create effective, safe topical solutions for healthcare and pharma distributors and brands. Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Menthol Gel Pain Relief Patch
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
















