To understand the pharmacokinetic profile of transdermal clonidine, it's essential to see it as a slow-onset, long-acting system. After the first application, it takes 2-3 days to achieve therapeutic blood levels. Once the patch is removed, plasma concentrations remain stable for about 8 hours before declining slowly, with a half-life of approximately 20-21 hours.
The core principle of transdermal clonidine is to mimic a continuous infusion, providing stable drug levels over a seven-day period. This avoids the peaks and troughs of oral dosing, reducing side effects but necessitating a slow start and a prolonged washout period.
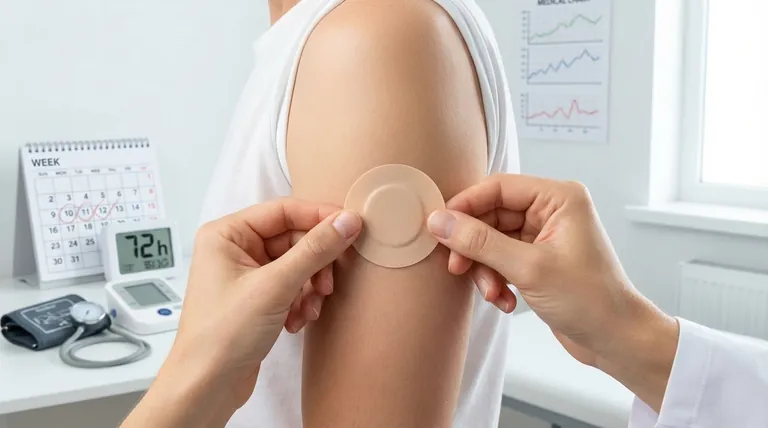
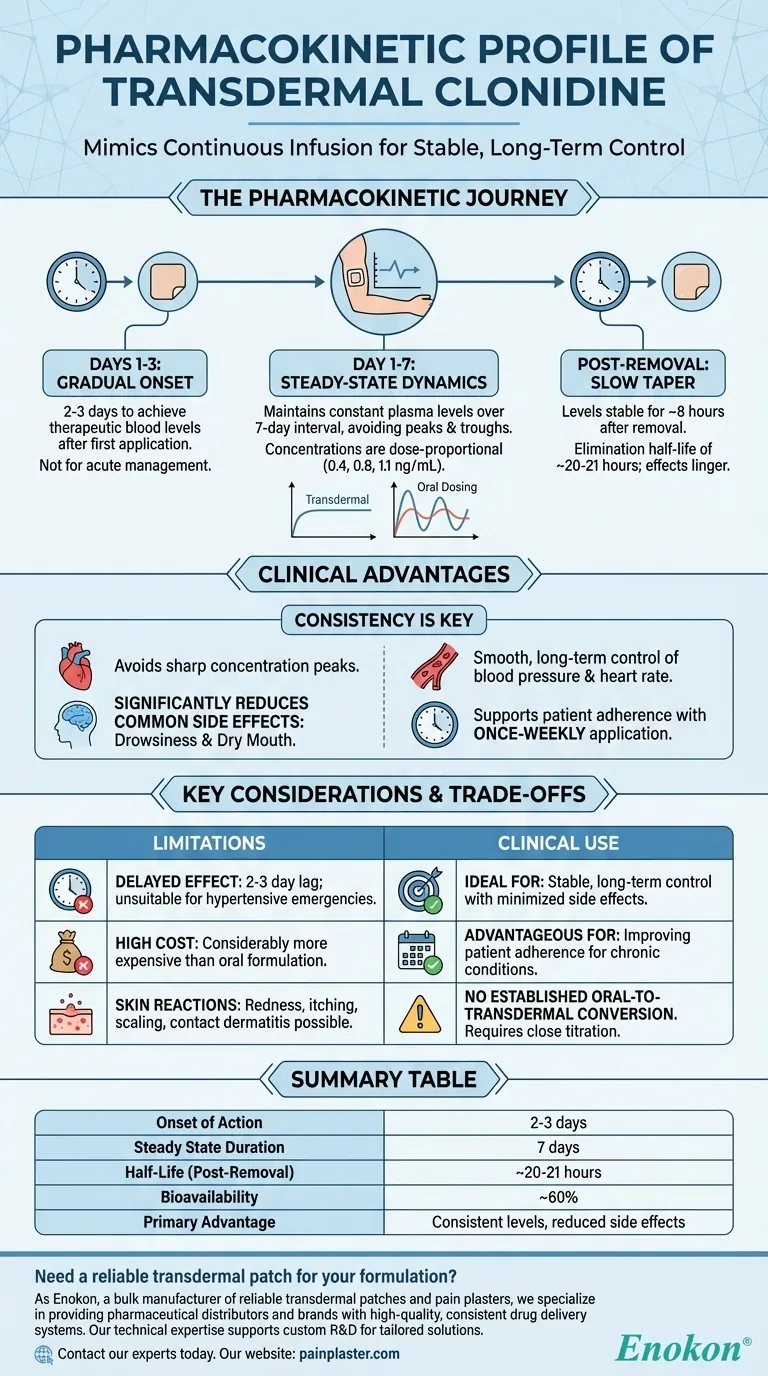
The Pharmacokinetic Journey of Transdermal Clonidine
The transdermal patch releases clonidine at a constant rate, which defines its unique clinical behavior. Understanding this journey from application to removal is key to using it effectively.
Onset of Action: A Gradual Climb
Unlike oral medications, the transdermal system does not produce an immediate effect. It takes approximately two to three days for the drug to absorb through the skin and reach a stable, therapeutic concentration in the bloodstream.
This slow onset means the patch is not suitable for acute management of hypertension but is designed for long-term, stable control.
Steady-State Dynamics: Infusion-Like Stability
Once steady-state is achieved, the patch delivers clonidine consistently, maintaining constant plasma levels throughout the 7-day dose interval. This pharmacokinetic pattern is highly desirable as it avoids the sharp peaks and troughs associated with oral therapy.
Mean steady-state concentrations are dose-proportional, reaching approximately 0.4, 0.8, and 1.1 ng/mL for the 0.1, 0.2, and 0.3 mg/day patch systems, respectively.
Elimination Profile: A Slow Taper
After a patch is removed, the drug that has accumulated in the skin layers continues to be absorbed. Plasma levels remain constant for about 8 hours post-removal.
Following this period, the concentration begins to decline with a half-life of around 20 to 21 hours. This prolonged elimination phase means the drug's effects will linger for several days after discontinuation.
Bioavailability and Excretion
Transdermal clonidine has an absolute bioavailability of about 60%. The body processes and eliminates the drug efficiently, with approximately 40-60% of the absorbed dose being excreted unchanged in the urine within 24 hours.
From Kinetics to Clinical Practice
This unique pharmacokinetic profile directly translates into specific clinical advantages and considerations for patient management.
The Primary Advantage: Consistency
The stable drug levels are the system's main therapeutic benefit. By avoiding concentration peaks, the patch significantly reduces common side effects like drowsiness and dry mouth that are often problematic with oral clonidine.
This consistency helps relax blood vessels and decrease heart rate more smoothly over the long term.
Application and Dosing
Patches are designed to deliver a specific dose (0.1, 0.2, or 0.3 mg per day) over a seven-day period. They should be applied to a clean, hairless area on the upper arm or torso, and application sites should be rotated weekly.
Crucially, there is no established oral-to-transdermal dose conversion, which means initiating patch therapy requires close blood pressure monitoring to titrate to the correct dose.
Understanding the Trade-offs
While effective, the transdermal system is not without its limitations. Its benefits must be weighed against its practical and financial costs.
The Delay in Therapeutic Effect
The 2-3 day lag time to reach therapeutic levels is a significant drawback. It makes the patch entirely unsuitable for hypertensive emergencies or any situation requiring rapid blood pressure reduction.
The High Cost Factor
A major barrier to the widespread use of transdermal clonidine is its considerably higher cost compared to the oral formulation. This economic factor often limits its use despite its clear therapeutic advantages in stability and side effect profile.
Potential for Skin Reactions
Direct contact with the patch can cause local skin reactions. These may include redness (erythema), itching (pruritus), scaling, blistering, and changes in skin pigmentation. Allergic contact dermatitis is a known issue, occurring more commonly in women and individuals with fair skin.
Making the Right Choice for Your Goal
Selecting between transdermal and oral clonidine depends entirely on the clinical objective and patient context.
- If your primary focus is stable, long-term blood pressure control with minimized side effects: The transdermal patch is an excellent option due to its consistent, infusion-like drug delivery.
- If your primary focus is patient adherence for chronic conditions: The simple, once-weekly application of the patch can be a significant advantage over multiple daily pills.
- If your primary focus is rapid blood pressure reduction or cost-effectiveness: Oral clonidine remains the more appropriate and accessible choice.
Ultimately, leveraging the transdermal clonidine patch effectively requires a clear understanding of its slow-on, slow-off kinetic profile.
Summary Table:
| Key Pharmacokinetic Parameter | Profile of Transdermal Clonidine |
|---|---|
| Onset of Action | 2-3 days to reach therapeutic levels |
| Steady State | Maintains stable levels over 7 days |
| Half-Life (Post-Removal) | ~20-21 hours |
| Bioavailability | ~60% |
| Primary Advantage | Avoids peaks/troughs, reduces side effects |
Need a reliable transdermal patch for your formulation?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we specialize in providing pharmaceutical distributors and brands with high-quality, consistent drug delivery systems. Our technical expertise supports custom R&D and development to create solutions tailored to your specific API and pharmacokinetic goals.
Contact our experts today to discuss how we can partner on your next transdermal project.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Menthol Gel Pain Relief Patch
People Also Ask
- How does the cough relief patch provide targeted relief? Direct, Soothing Comfort for Coughs & Chest Congestion
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- How does the far infrared technology in the cough relief patch work? Enhance Natural Ingredient Delivery
- What types of coughs can the far infrared cough relief patch address? Soothe Dry, Wet, and Persistent Coughs
- What makes the cough relief patch a convenient option for managing coughs? A Mess-Free, On-the-Go Solution

















