A transdermal Alzheimer's vaccine represents an innovative approach to preventing the disease by targeting beta-amyloid protein buildup in the brain through skin-based delivery. Early studies in mice show potential for clearing these harmful proteins without the inflammatory side effects associated with traditional injection-based vaccines. However, the technology is still in preclinical stages, and human trials are needed to confirm safety and efficacy. If successful, this method could offer a non-invasive, patient-friendly alternative to current Alzheimer's treatments, though it may take several years before it becomes clinically available.

Key Points Explained:
-
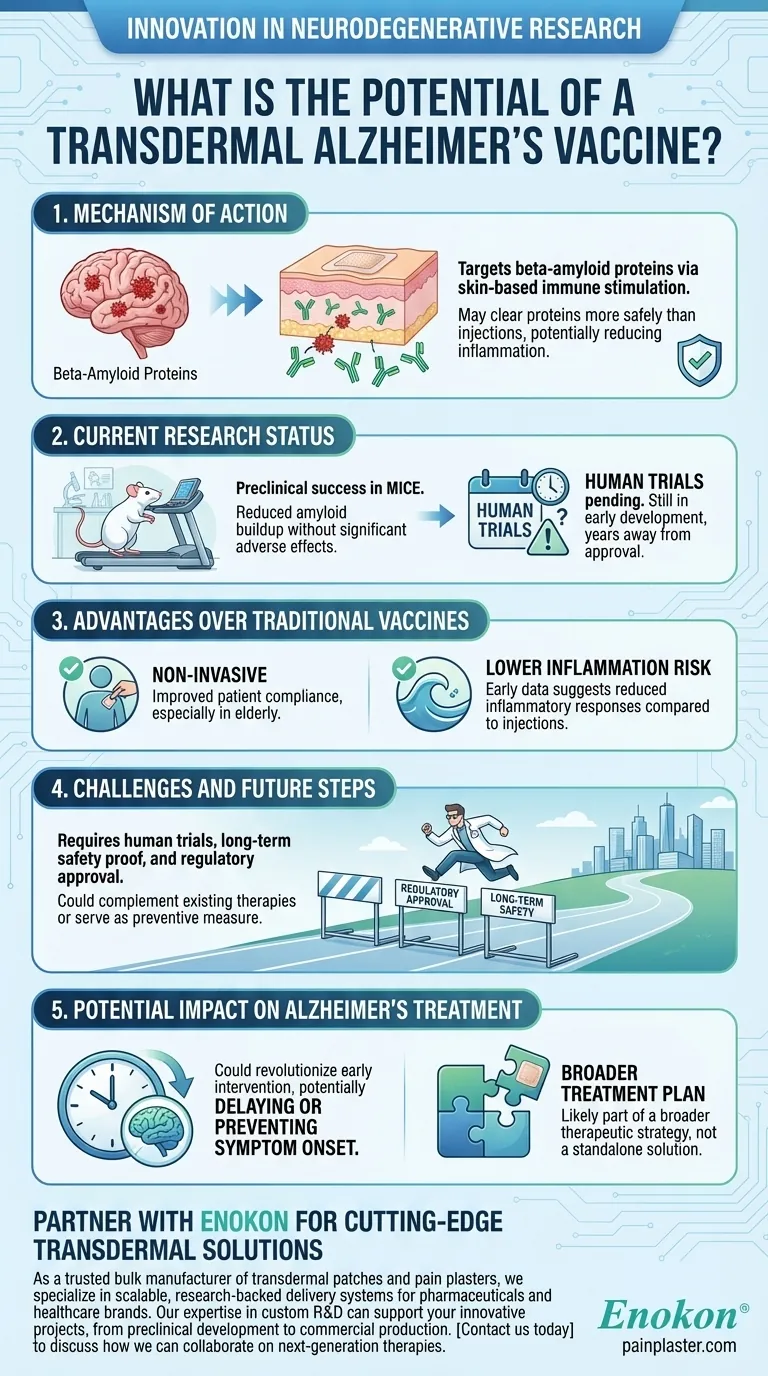
Mechanism of Action
- The vaccine targets beta-amyloid proteins, which are believed to play a key role in Alzheimer's disease progression by forming plaques in the brain.
- Transdermal delivery (through the skin) may stimulate an immune response that clears these proteins more safely than injections, which have historically caused inflammation in some patients.
-
Current Research Status
- Preclinical trials in mice have shown promising results in reducing amyloid buildup without significant adverse effects.
- Human trials have not yet begun, meaning the vaccine is still in early development and years away from potential approval.
-
Advantages Over Traditional Vaccines
- Non-invasive administration could improve patient compliance, especially in elderly populations who may struggle with frequent injections.
- Early data suggests reduced risk of inflammatory responses compared to past injection-based Alzheimer's vaccines.
-
Challenges and Future Steps
- Scaling from animal models to humans involves significant regulatory and scientific hurdles, including proving long-term safety and efficacy.
- If successful, this approach could complement existing therapies or serve as a preventive measure for at-risk individuals.
-
Potential Impact on Alzheimer's Treatment
- A transdermal vaccine could revolutionize early intervention strategies, potentially delaying or preventing symptom onset.
- However, given the complexity of Alzheimer's disease, it may not be a standalone solution but part of a broader therapeutic strategy.
This development highlights the ongoing innovation in neurodegenerative disease research, offering hope for more accessible and tolerable treatments in the future.
Summary Table:
| Key Aspect | Details |
|---|---|
| Mechanism of Action | Targets beta-amyloid proteins via skin-based immune stimulation. |
| Current Research Status | Preclinical success in mice; human trials pending. |
| Advantages | Non-invasive, lower inflammation risk, better patient compliance. |
| Challenges | Requires human trials, long-term safety proof, and regulatory approval. |
| Potential Impact | Could delay/prevent Alzheimer's onset as part of a broader treatment plan. |
Partner with Enokon for cutting-edge transdermal solutions
As a trusted bulk manufacturer of transdermal patches and pain plasters, we specialize in scalable, research-backed delivery systems for pharmaceuticals and healthcare brands. Our expertise in custom R&D can support your innovative projects, from preclinical development to commercial production.
Contact us today to discuss how we can collaborate on next-generation therapies.
Visual Guide
Related Products
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- What are the physical characteristics of a Pain Relief Patch? Designed for Discreet, All-Day Comfort
- What are the key components of a pain relief patch? Unlock the Science of Targeted Pain Relief
- What are the benefits of using a pain relief patch instead of oral medication? Get Targeted, Long-Lasting Relief
- What are the benefits of Pain Relief Patch being licensed as a medicine? Guaranteed Efficacy & Safety
















