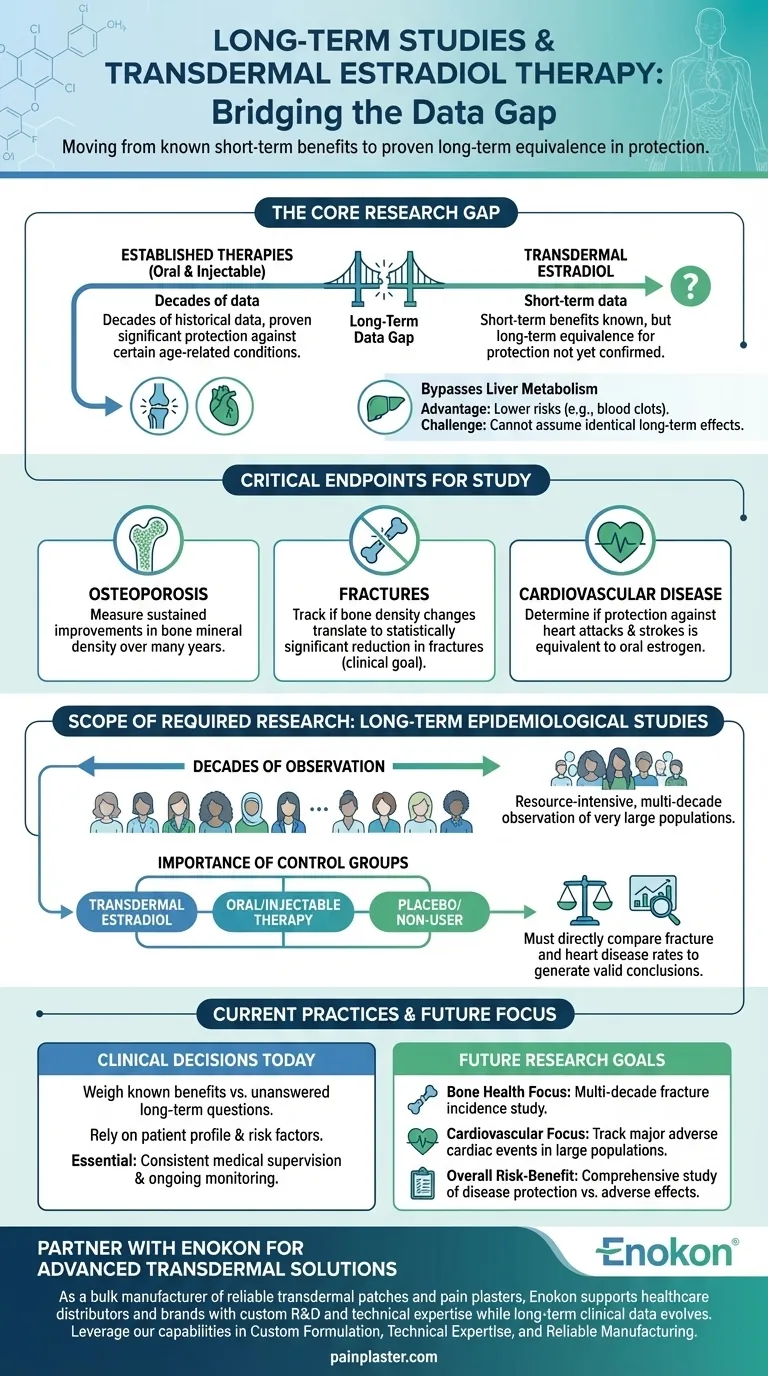
To be clear, long-term epidemiological studies are needed to determine if transdermal estradiol provides the same level of protection against osteoporosis, fractures, and cardiovascular disease that has been established for oral and injectable forms of estrogen. These studies are essential for validating its role as an equivalent long-term therapeutic option.
The central challenge is moving from known short-term benefits to proven long-term outcomes. While transdermal estradiol has clear advantages in how it's metabolized, large-scale, multi-decade data is required to confirm its protective effects on bone and heart health are equal to other, more established estrogen therapies.

The Core Research Gap: Proving Long-Term Equivalence
The fundamental question is not whether transdermal estradiol is effective, but whether its long-term protective outcomes match those of therapies with decades of historical data.
Why a Comparison is Necessary
Oral and injectable estrogens have been studied for many years. This research has established their ability to offer significant protection against certain age-related conditions.
Transdermal estradiol functions differently, primarily by bypassing the liver's first-pass metabolism. This is a significant advantage for reducing certain risks, but it also means we cannot automatically assume its long-term protective mechanisms and effects are identical.
The Specific Endpoints for Study
Large-scale epidemiological studies must focus on three critical health outcomes:
- Osteoporosis: Measuring if transdermal use leads to sustained improvements in bone mineral density over many years.
- Fractures: Tracking whether the changes in bone density translate into a statistically significant reduction in actual bone fractures, which is the ultimate clinical goal.
- Cardiovascular Disease: Determining if transdermal estradiol provides a level of protection against heart attacks, strokes, and other cardiovascular events that is equivalent to that seen with oral estrogen.
Understanding the Scope of the Required Research
"Long-term epidemiological studies" are a specific and resource-intensive type of research needed to answer these questions definitively.
What Defines an Epidemiological Study
This involves observing very large populations over extended periods, often decades. Researchers track the incidence of disease and compare outcomes between groups using different treatments, like transdermal versus oral estrogen, or no treatment at all.
The Importance of Control Groups
To generate valid conclusions, these studies must directly compare cohorts of patients. It is essential to measure the rates of fractures or heart disease in women using transdermal estradiol against those using oral therapy, injectable therapy, and a placebo or non-user group.
Current Practices and Necessary Precautions
While this definitive long-term data is being gathered, healthcare providers must rely on existing evidence and careful patient management.
Making Decisions with Current Data
Clinicians weigh the known benefits of transdermal delivery—such as a potentially lower risk of blood clots—against the unanswered questions about long-term equivalence for bone and heart protection. The choice of therapy is based on an individual patient's health profile and risk factors.
The Critical Role of Ongoing Monitoring
The current gaps in long-term data underscore the importance of consistent medical supervision for any patient on hormone therapy.
Regular physical exams, including pelvic exams, and relevant lab tests are not a substitute for research, but they are essential tools to monitor the treatment's effects and ensure patient safety on an individual basis.
Making the Right Choice for Future Research
To close the current knowledge gap, future research must be highly focused and goal-oriented.
- If the primary focus is bone health: The key is a multi-decade study tracking fracture incidence as the primary outcome, comparing transdermal estradiol directly against oral forms.
- If the primary focus is cardiovascular protection: Studies must be designed to track major adverse cardiac events in large, diverse populations of women using transdermal therapy versus other methods.
- If the primary focus is overall risk-benefit analysis: Comprehensive studies are needed that simultaneously track protection against disease and the incidence of potential adverse effects over a patient's lifetime.
Ultimately, these dedicated long-term studies are the only way to fully confirm the place of transdermal estradiol in preventative medicine for women.
Summary Table:
| Key Research Areas | Primary Endpoints | Comparison Groups |
|---|---|---|
| Osteoporosis & Fractures | Bone mineral density; Fracture incidence | vs. Oral/Injectable Estrogen; Placebo |
| Cardiovascular Disease | Heart attacks; Strokes; Cardiac events | vs. Oral/Injectable Estrogen; Placebo |
| Overall Risk-Benefit | Disease protection; Adverse effects | Long-term, multi-decade observation |
Partner with Enokon for Advanced Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon supports healthcare and pharmaceutical distributors and brands in navigating complex therapeutic landscapes. Our technical expertise in custom R&D and development can help you create innovative hormone therapy solutions while the long-term clinical data evolves.
Benefit from our capabilities:
- Custom Formulation: Tailor transdermal delivery systems to specific therapeutic needs.
- Technical Expertise: Leverage our deep knowledge of transdermal technology for your product development.
- Reliable Manufacturing: Scale your production with a trusted partner for consistent, high-quality patches.
Ready to develop or enhance your transdermal product line? Contact our experts today to discuss how we can support your goals with reliable manufacturing and expert R&D collaboration.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
- Capsaicin Chili Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief













