Before using a granisetron transdermal patch, it is crucial to inform your doctor about any history of stomach or intestinal disorders, recent gastrointestinal surgery, or previous allergic reactions to any type of medicated skin patch. These conditions are the most direct and serious contraindications for this specific medication.
Your complete medical history allows your physician to weigh the benefits of granisetron against potential risks. The primary concern is the medication's ability to mask the symptoms of a serious underlying gastrointestinal blockage.
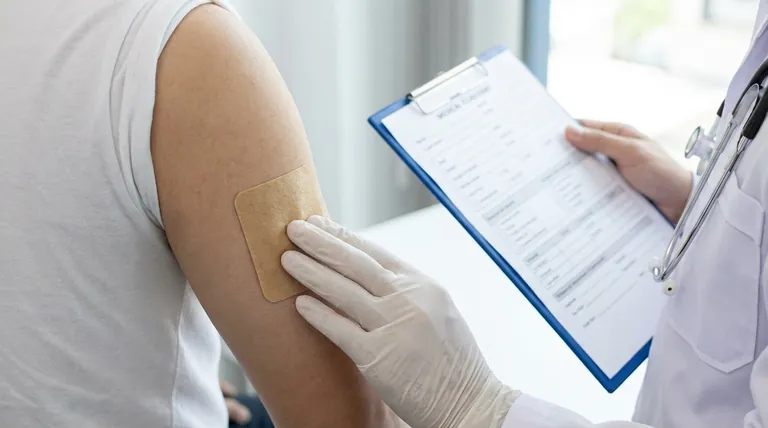
Core Conditions of Concern for Granisetron
To ensure your safety, your doctor must be aware of specific conditions that can interact negatively with how the granisetron patch works or is delivered through your skin.
Gastrointestinal Health
Your stomach and intestinal history is the most critical piece of information. Granisetron works by preventing nausea and vomiting, which are also key symptoms of a bowel obstruction.
Informing your doctor about any stomach or intestinal disorders (like ulcers, bleeding, or chronic inflammatory conditions) is vital.
Likewise, you must disclose any recent stomach or intestinal surgery, as this increases the risk of complications that granisetron could inadvertently hide.
History of Skin Allergies
The delivery system of the medication—a transdermal patch—is as important as the drug itself.
Disclose any history of allergic reactions to medicated skin patches of any kind. A past reaction, even to a different medication, indicates a higher sensitivity to the adhesives or components used in transdermal systems.
Broader Medical History to Disclose
While gastrointestinal issues are the top priority, a complete medical picture helps prevent other potential complications. Providing a full history ensures the highest level of safety.
Neurological and Organ Health
Certain conditions can affect how your body processes medication or may be aggravated by it.
Be sure to mention any history of seizures, as some medications can influence seizure thresholds.
Disclosing liver or kidney disease is also important. These organs are responsible for metabolizing and clearing drugs from your system, and any impairment could alter the medication's effects.
Heart and Circulatory Conditions
While not the primary area of concern for granisetron, any pre-existing heart problems should be part of the conversation with your doctor to ensure a comprehensive safety assessment.
Understanding the Primary Risk: Masking Symptoms
The most significant danger associated with granisetron is not a direct side effect but its potential to hide a developing medical emergency.
The Danger of a Masked Obstruction
Granisetron is a potent anti-nausea medication. A serious condition known as ileus, or decreased intestinal movement, can lead to a bowel blockage.
Because granisetron effectively stops vomiting, it can mask this key symptom, causing a dangerous delay in the diagnosis and treatment of a bowel obstruction. This is why your gastrointestinal history is paramount.
Potential for Skin Reactions
Transdermal patches can cause application site reactions, such as redness, itching, or rash. While often mild, a history of sensitivity to adhesives or other patches increases the likelihood of a more severe reaction.
Making the Right Choice for Your Goal
A transparent conversation with your healthcare provider is the most effective way to ensure a safe and successful treatment.
- If your primary concern is preventing a serious complication: Be absolutely explicit about any past or present stomach surgeries, intestinal blockages, or chronic conditions like Crohn's disease.
- If you have sensitive skin or a history of allergies: Ask your doctor about monitoring for skin reactions and what alternative options may be available if one occurs.
- If you manage multiple health conditions: Provide a complete list of all your diagnoses and the medications you take, including supplements, to prevent any unforeseen interactions.
Ultimately, empowering your doctor with your complete medical history is the best way to protect your own health.
Summary Table:
| Condition Category | Specific Conditions to Disclose |
|---|---|
| Gastrointestinal Health | Stomach/intestinal disorders, recent GI surgery, chronic conditions (e.g., Crohn's disease). |
| Allergy History | Previous allergic reactions to any medicated skin patches or adhesives. |
| Organ & Neurological Health | Liver or kidney disease, history of seizures. |
| Other Considerations | Heart conditions, complete list of current medications and supplements. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Ensure the highest standards of safety and efficacy for your patients.
Contact our experts today to discuss your custom transdermal solution.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief












