In clinical studies, other allergic reactions reported with the clonidine transdermal system included maculopapular rash, urticaria (hives), and angioedema (swelling) of the face. It is important to note, however, that a direct causal relationship to the medication was not definitively established for these specific cases.
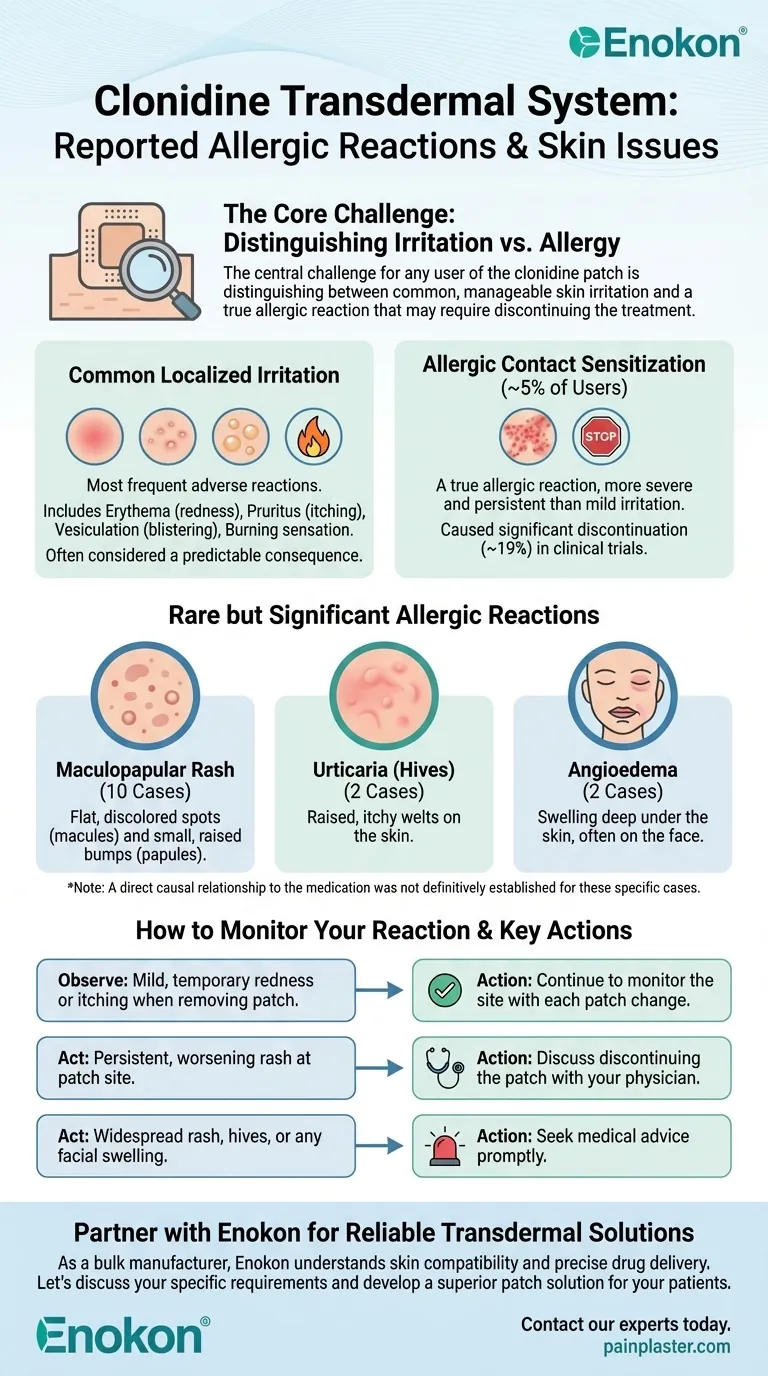
The central challenge for any user of the clonidine patch is distinguishing between common, manageable skin irritation and a true allergic reaction that may require discontinuing the treatment. Understanding this difference is key to using the system safely and effectively.
Differentiating Skin Reactions: Irritation vs. Allergy
Most adverse reactions to the clonidine patch occur on the skin at the application site. These can be broadly categorized into common irritation and less common but more significant allergic sensitization.
Common Localized Irritation
The most frequent side effects are dermatological. Studies show that a significant number of patients experience localized skin reactions.
These common irritations include redness (erythema), itching (pruritus), blistering (vesiculation), and a burning sensation. These are often considered a predictable consequence of wearing an adhesive patch.
Allergic Contact Sensitization
A smaller but still notable percentage of users (around 5%) develop allergic contact sensitization. This is a true allergic reaction, not just simple irritation.
This condition, often called contact dermatitis, is more severe and persistent than mild irritation. It was significant enough that approximately 19% of all patients in clinical trials discontinued treatment because of it.
Rare but Significant Allergic Reactions
Beyond localized contact dermatitis, clinical trials recorded a few other specific types of allergic reactions. These were much less common.
Maculopapular Rash
This was the most frequently reported of the rarer allergic reactions, with 10 cases noted. A maculopapular rash is characterized by a combination of flat, discolored spots (macules) and small, raised bumps (papules).
Urticaria (Hives)
There were 2 cases of urticaria reported. Urticaria, commonly known as hives, appears as raised, itchy welts on the skin.
Angioedema
Two cases of angioedema of the face were also documented. Angioedema is a more serious reaction involving swelling deep under the skin, often around the eyes and lips.
Understanding the Broader Context
It is helpful to separate the skin-related side effects from the systemic ones, which affect the rest of the body.
Local vs. Systemic Effects
While skin reactions are the most common issue, clonidine is absorbed into the bloodstream to work. This can cause systemic side effects that are unrelated to an allergy.
The most common systemic effects are dry mouth (affecting 25% of patients) and drowsiness (12%). Other effects include fatigue, headache, dizziness, and constipation. These are caused by the drug's action on the body, not a skin reaction.
The Causality Caveat
For the rare reports of maculopapular rash, urticaria, and angioedema, researchers could not definitively prove the clonidine patch was the cause. This uncertainty is a crucial piece of context.
How to Monitor Your Reaction
Given the high rate of skin issues, from mild irritation to allergic dermatitis, proactive monitoring is essential.
When to Observe vs. When to Act
Mild, temporary redness or itching when you remove a patch can be normal. However, a reaction that worsens, spreads, or persists may indicate a developing allergic sensitization.
Severe reactions, such as widespread rash, hives, or any facial swelling, are signs that require immediate medical consultation. The high discontinuation rate (19%) due to contact dermatitis shows that stopping the medication is a common and necessary outcome for many patients.
Key Actions for Patients
Based on the clinical data, here is how to approach potential skin reactions:
- If you experience mild, temporary redness or itching: This is a very common reaction; continue to monitor the site with each patch change.
- If you develop a persistent, worsening rash at the patch site: You may be developing allergic contact dermatitis and should discuss discontinuing the patch with your physician.
- If you notice a widespread rash, hives, or any swelling of the face: This indicates a more significant potential allergic reaction, and you should seek medical advice promptly.
Ultimately, being an informed and observant patient is the most effective way to manage your treatment and recognize when a reaction requires professional medical guidance.
Summary Table:
| Reaction Type | Reported Cases | Key Characteristics |
|---|---|---|
| Maculopapular Rash | 10 | Flat, discolored spots and small, raised bumps. |
| Urticaria (Hives) | 2 | Raised, itchy welts on the skin. |
| Angioedema | 2 | Swelling deep under the skin, often on the face. |
| Allergic Contact Sensitization | ~5% of users | Persistent, worsening rash at application site. |
Need a reliable, hypoallergenic transdermal patch solution? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand the critical importance of skin compatibility and precise drug delivery. Our technical expertise in custom R&D and development helps healthcare and pharma brands create safer, more effective products. Let's discuss your specific requirements and develop a superior patch solution for your patients. Contact our experts today for a consultation.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














