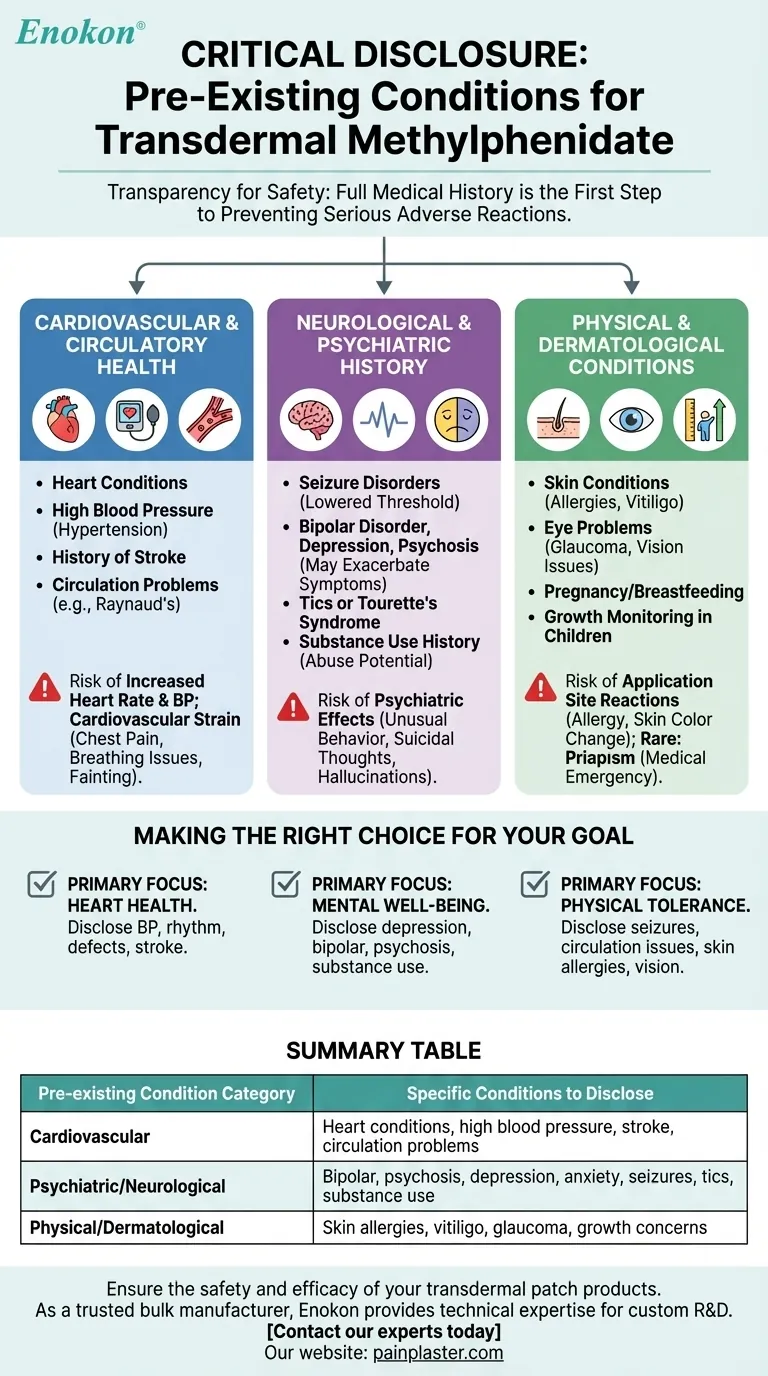
Before using transdermal methylphenidate, you must provide your doctor with a complete medical history. This includes any personal or family history of heart conditions, high blood pressure, circulation problems, mental health disorders like bipolar or psychosis, seizure disorders, tics or Tourette's syndrome, substance use, and specific skin or eye conditions. It is also critical to disclose if you are pregnant, planning to become pregnant, or breastfeeding.
The core principle is transparency for safety. Methylphenidate is a stimulant that can significantly impact the cardiovascular, neurological, and psychiatric systems, making a full disclosure of your medical history the most critical step in preventing serious adverse reactions.

Cardiovascular and Circulatory Health
Stimulant medications directly affect the heart and blood vessels. Disclosing your full history in this area is non-negotiable for safe use.
Heart Conditions and Blood Pressure
Methylphenidate can increase both heart rate and blood pressure. For individuals with pre-existing structural heart abnormalities, high blood pressure (hypertension), or a history of stroke, this added strain can be dangerous.
Circulation Problems
You must report conditions like Raynaud phenomenon or other peripheral vasculopathies. The medication can constrict blood vessels, potentially worsening symptoms such as numbness, coldness, or pain in the fingers and toes.
Neurological and Psychiatric History
Methylphenidate acts on the central nervous system. Its effects can profoundly interact with underlying neurological and mental health conditions.
Seizure Disorders
This medication can lower the convulsive threshold, which means it may increase the risk of seizures in individuals with a prior history of them. Full disclosure allows your doctor to weigh this risk carefully.
Mental Health Conditions
For those with a history of bipolar disorder, depression, or psychosis, methylphenidate can sometimes exacerbate symptoms. It may trigger manic episodes, agitation, or even hallucinations, making psychiatric stability a key factor in prescribing.
Tics and Tourette's Syndrome
Stimulants are known to unmask or worsen both motor and verbal tics. A personal or family history of tics or Tourette's syndrome is a critical piece of information for your prescriber.
History of Substance Use
Because methylphenidate is a stimulant with a potential for abuse and dependence, any personal or family history of alcohol or drug abuse must be discussed openly.
Physical and Dermatological Conditions
The transdermal patch delivery system introduces unique considerations for skin health, while the medication itself can affect other physical systems.
Skin Conditions
You must report any history of skin allergies, especially to medicated patches, as well as conditions like vitiligo. The patch can cause contact sensitization or a persistent loss of skin pigmentation at the application site.
Eye Problems
Disclose any history of glaucoma or other serious eye conditions. Methylphenidate can sometimes cause blurry vision or difficulties with visual accommodation.
Growth Monitoring in Children
In pediatric patients, stimulants can potentially slow growth. This is a known side effect that requires regular monitoring of height and weight by a physician.
Understanding the Key Risks
Disclosing your history is about managing risk. Certain serious side effects, while rare, are directly linked to these pre-existing conditions.
Cardiovascular Strain
The primary risk is to the heart. This can manifest as chest pain, trouble breathing, or fainting, which require immediate medical attention.
Psychiatric Effects
Be aware of the potential for unusual changes in behavior, suicidal thoughts, or hallucinations. These are serious psychiatric side effects that must be reported instantly.
Application Site Reactions
The skin is a key area of concern with the patch. Beyond simple irritation, watch for signs of allergic reaction or permanent changes in skin color.
Other Serious Reactions
A rare but serious side effect is priapism, a painful erection lasting more than four hours. This is a medical emergency that requires immediate care.
Making the Right Choice for Your Goal
Full disclosure is the foundation of safe and effective treatment. Use this checklist to ensure you have a comprehensive conversation with your healthcare provider.
- If your primary focus is heart health: You must disclose any personal or family history of high blood pressure, heart rhythm problems, structural heart defects, or stroke.
- If your primary focus is mental well-being: It is essential to discuss any history of depression, bipolar disorder, psychosis, anxiety, or substance use disorder.
- If your primary focus is physical tolerance: Be sure to mention any history of seizures, circulation issues like Raynaud's, skin allergies, or vision problems.
Proactive and honest communication with your doctor is the most effective tool you have to ensure your treatment is both safe and successful.
Summary Table:
| Pre-existing Condition Category | Specific Conditions to Disclose |
|---|---|
| Cardiovascular | Heart conditions, high blood pressure, stroke, circulation problems (e.g., Raynaud's) |
| Psychiatric/Neurological | Bipolar disorder, psychosis, depression, anxiety, seizures, tics/Tourette's, substance use |
| Physical/Dermatological | Skin allergies, vitiligo, glaucoma, growth concerns (in children) |
Ensure the safety and efficacy of your transdermal patch products.
As a trusted bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. We help you create safe, effective solutions for your patients.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- Who should consult a healthcare professional before using pain relief patches? Ensure Your Safety with Medical Advice
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- What was the reported pain relief after the initial month of plaster use? Consistent & Effective Pain Management

















