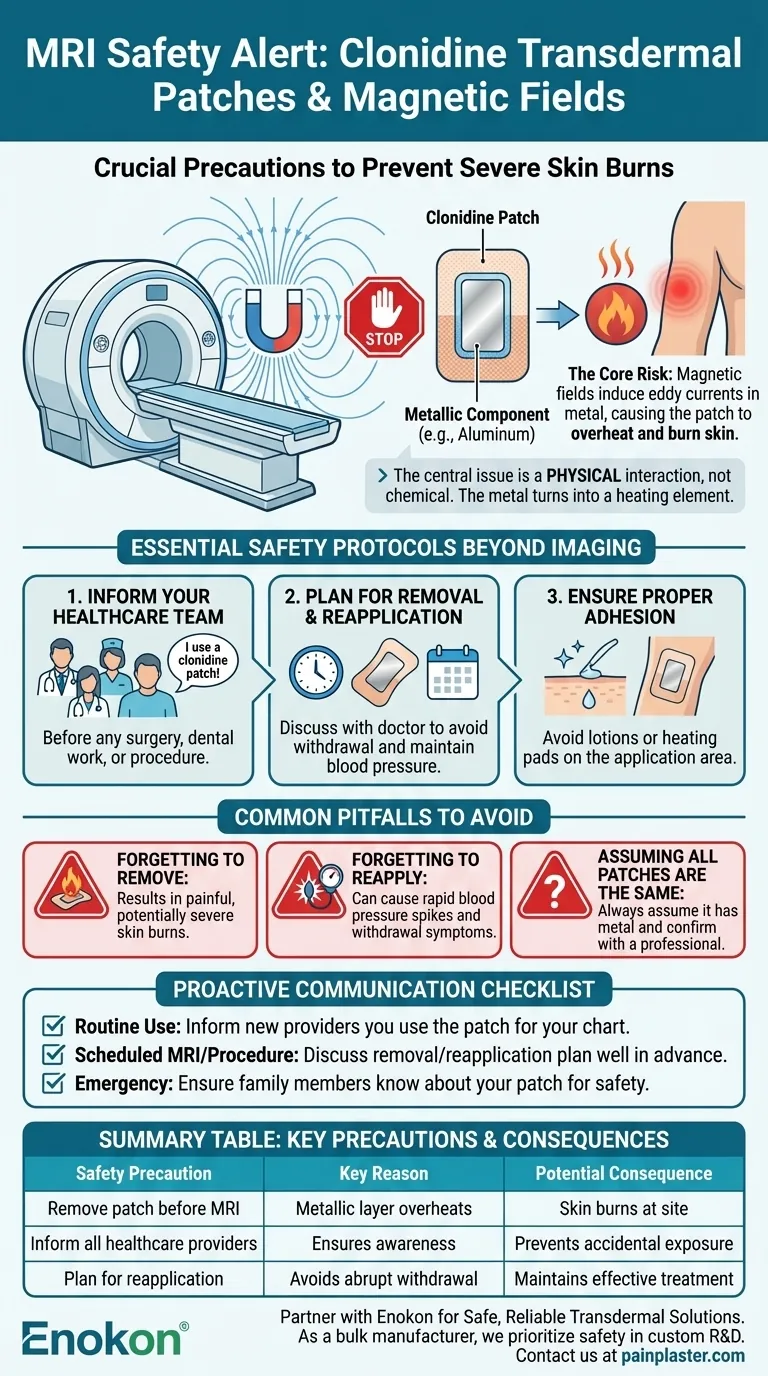
To be clear, the clonidine transdermal patch must be removed before you undergo a Magnetic Resonance Imaging (MRI) scan. The patch contains a metallic component, typically aluminum, which can overheat when exposed to the MRI's powerful magnetic fields. This poses a significant risk of causing serious skin burns at the application site.
The central issue is not a chemical reaction but a physical one. The metallic layer in many transdermal patches can interact with an MRI's magnetic field, turning the patch into a heating element and creating a direct risk of burning your skin.
The Core Risk: Why Patches and MRIs Don't Mix
Understanding the "why" behind this precaution is critical for ensuring your safety during medical procedures. The interaction is a straightforward matter of physics.
The Power of Magnetic Fields
An MRI machine uses powerful magnets and radio waves to create detailed images of the inside of your body. These magnetic fields are strong enough to interact with any metallic objects.
How a Burn Occurs
The clonidine patch contains a thin layer of aluminum. When this metal is exposed to the MRI's magnetic field, it can induce an electrical current. This phenomenon, known as an eddy current, rapidly generates heat, which can cause a first-degree or even second-degree burn on the skin directly beneath the patch.
Not Just for MRIs
This same risk applies to other medical procedures involving powerful electrical currents. The patch should also be removed before defibrillation or cardioversion to prevent burns and ensure the equipment functions correctly.
Essential Safety Protocols Beyond Imaging
While the MRI precaution is critical, it's part of a broader set of safety measures for using the clonidine patch effectively.
Always Inform Your Healthcare Team
Your most important responsibility is communication. Before any surgery, dental work, or medical procedure, you must inform every doctor, nurse, and technician that you are using a clonidine transdermal patch.
Plan for Removal and Reapplication
Simply removing the patch is only half the battle. Discuss with your prescribing doctor the plan for reapplication. You will need to know where to apply a new patch and how soon after the procedure it should be done to maintain stable blood pressure control.
Ensure Proper Adhesion
The effectiveness of the patch depends on it sticking properly. Avoid using lotions or oils on the application area. Additionally, do not cover the patch with a heating pad, as this can increase drug absorption to dangerous levels.
Understanding the Common Pitfalls
Navigating medical care with a transdermal patch requires diligence. Being aware of common mistakes can help you avoid potential harm.
Forgetting to Remove the Patch
The primary consequence of this mistake during an MRI or defibrillation is a painful and potentially severe skin burn. There are no exceptions to this rule.
Forgetting to Reapply the Patch
Failing to reapply a new patch after your procedure can be just as dangerous. Abruptly stopping clonidine can cause a rapid increase in blood pressure and withdrawal symptoms. Always follow your doctor's instructions for reapplication.
Assuming All Patches Are the Same
This warning applies specifically to patches containing a metallic layer. While it is a common design, not all transdermal patches have one. However, it is always safest to assume it does and confirm with your pharmacist or doctor.
Making the Right Choice for Your Goal
Proactive communication is the key to using a clonidine patch safely. Use this simple checklist to guide your conversations with medical staff.
- If your primary focus is routine use: Always inform any new healthcare provider that you use a clonidine patch so it is documented in your chart.
- If you have a scheduled MRI or procedure: Discuss your plan for removing and reapplying the patch with your doctor well in advance of your appointment.
- If you face an emergency: Medical personnel are trained to look for patches, but ensuring your family members know you use one can provide an extra layer of safety.
Ultimately, your safety depends on a clear partnership between you and your healthcare team.
Summary Table:
| Safety Precaution | Key Reason | Potential Consequence |
|---|---|---|
| Remove patch before MRI | Metallic layer (e.g., aluminum) can overheat | Skin burns at application site |
| Inform all healthcare providers | Ensures awareness before any procedure | Prevents accidental exposure to magnetic fields/currents |
| Plan for reapplication | Avoids abrupt withdrawal and blood pressure spikes | Maintains effective treatment post-procedure |
Partner with Enokon for Safe, Reliable Transdermal Solutions
As a bulk manufacturer of transdermal patches and pain plasters for healthcare and pharmaceutical distributors, we understand the critical importance of patient safety and product reliability. Our technical expertise in custom R&D and development ensures your patches are designed with the latest safety standards in mind.
Let us help you develop safe, effective transdermal products. Contact our experts today to discuss your needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
















