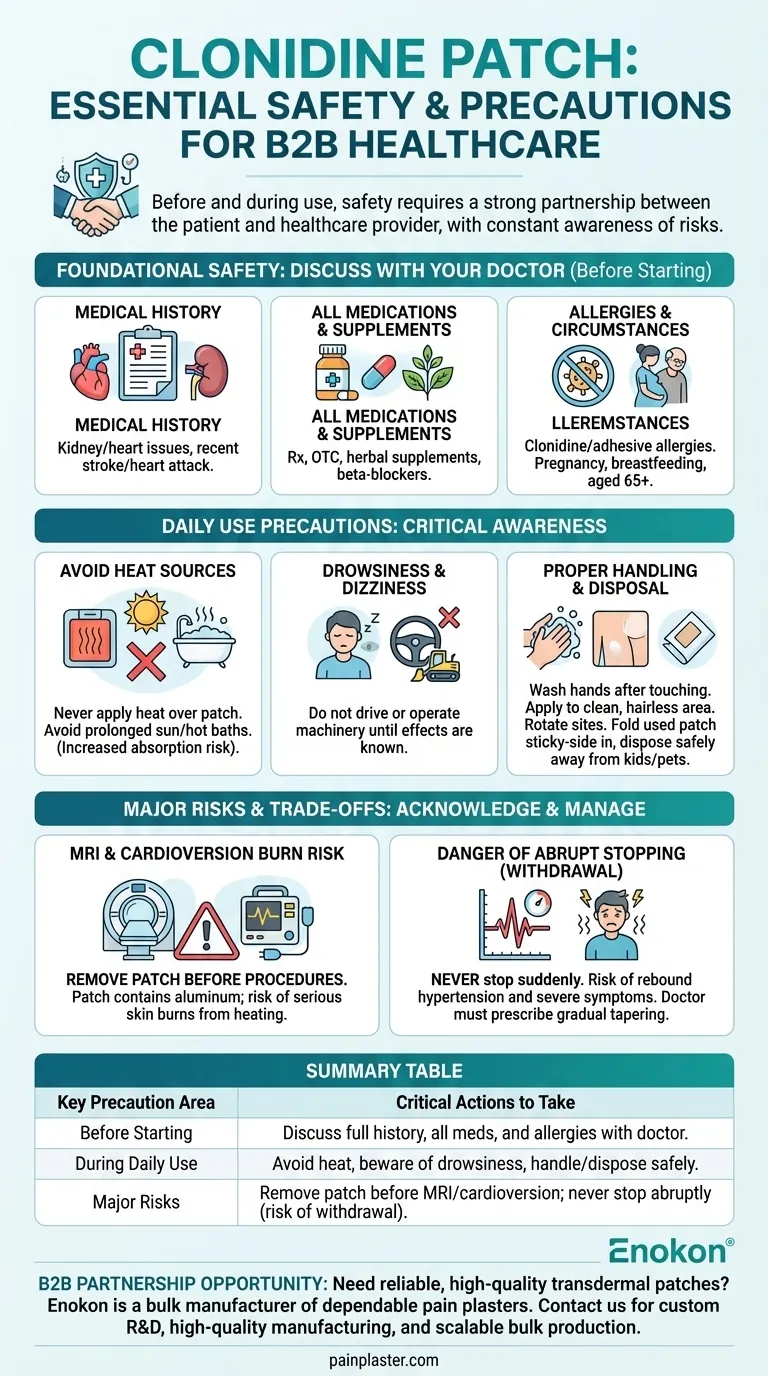
Before using a clonidine patch, you must have a thorough discussion with your doctor about your complete health profile. This includes your full medical history (especially heart or kidney issues), all other medications you are taking, and any known allergies. It is equally critical to understand the serious risks related to heat exposure, MRI procedures, and the dangers of suddenly stopping the medication.
The safe use of a clonidine patch is not just about correct application. It requires a partnership with your healthcare provider and constant awareness of how heat, medical procedures, and discontinuation can lead to severe health complications.

Foundational Safety: What to Discuss With Your Doctor
Before your first patch is ever applied, a comprehensive medical review is the most important precaution you can take. Your doctor needs a complete picture to ensure this medication is safe and appropriate for you.
Your Complete Medical History
Be transparent about any history of kidney disease, heart disease, a recent heart attack, or a past stroke. These conditions can significantly alter how your body responds to clonidine.
All Medications and Supplements
Provide a comprehensive list of everything you take. This includes all prescriptions, over-the-counter drugs, and herbal supplements. Drug interactions can increase side effects, and special care is needed if you take beta-blockers.
Known Allergies
Inform your doctor of any allergies you have, particularly to clonidine itself or any adhesives or ingredients used in transdermal patches.
Special Circumstances
You must discuss if you are pregnant, planning to become pregnant, or are currently breastfeeding. Also, inform your doctor if you are aged 65 or older, as your risk of side effects may be higher.
Critical Precautions During Daily Use
Once you begin treatment, safety depends on your daily habits and awareness of potential hazards.
Avoid All External Heat Sources
Never apply heat sources like heating pads or electric blankets over the patch site. You should also avoid prolonged, direct sun exposure or long, hot baths. Heat can dramatically increase the rate of medication absorption, potentially leading to an overdose.
The Drowsiness and Dizziness Factor
Clonidine often causes drowsiness, dizziness, or blurred vision. Do not drive a car or operate heavy machinery until you are certain how the medication affects you.
Proper Application and Handling
Always wash your hands thoroughly after touching a patch to avoid transferring potent medication to your eyes, nose, or mouth. Apply the patch to a clean, dry, and hairless area of skin, and be sure to rotate the application site with each new patch.
Safe Disposal of Used Patches
Even used patches contain a significant amount of residual medication. To dispose of one safely, fold it in half with the sticky sides pressed together and place it where children and pets cannot access it.
Understanding the Major Risks and Trade-offs
Using a clonidine patch involves acknowledging and managing a few significant risks. Ignoring these can have severe consequences.
The MRI and Cardioversion Burn Risk
The clonidine patch contains aluminum in its backing layer. You must remove the patch before undergoing an MRI scan or cardioversion. The metal can heat up during these procedures and cause serious skin burns at the patch site.
The Danger of Abruptly Stopping (Withdrawal)
Never stop using the clonidine patch suddenly or without your doctor's guidance. Abrupt discontinuation can trigger severe withdrawal symptoms, including a rapid and dangerous rise in blood pressure, known as rebound hypertension.
The Discontinuation Protocol
Other withdrawal symptoms can include nervousness, agitation, headache, and tremors. If you need to stop the medication, your doctor must prescribe a gradual tapering schedule, typically over two to four days, to prevent these effects.
How to Apply This to Your Goal
Your approach to safety will depend on where you are in your treatment journey.
- If your primary focus is preparing to start treatment: Your main task is to create a complete health profile for your doctor, listing every condition, medication, and allergy.
- If your primary focus is safe daily use: Your constant priority is to avoid heat exposure on the patch, remain cautious of drowsiness, and follow strict handling and disposal rules.
- If your primary focus is planning for a procedure or stopping medication: You must proactively inform all medical staff about the patch before an MRI or surgery and never attempt to stop treatment without a doctor's tapering plan.
By treating this medication with informed caution, you empower yourself to manage your condition safely and effectively.
Summary Table:
| Key Precaution Area | Critical Actions to Take |
|---|---|
| Before Starting | Discuss full medical history, all medications, and allergies with your doctor. |
| During Daily Use | Avoid heat on the patch, beware of drowsiness, and handle/dispose of patches safely. |
| Major Risks | Remove patch before MRI/cardioversion; never stop treatment abruptly (risk of withdrawal). |
Need a reliable, high-quality transdermal patch for your healthcare brand or distribution network?
At Enokon, we are a bulk manufacturer of dependable transdermal patches and pain plasters. We understand the critical importance of safety, precise dosing, and consistent performance in medications like clonidine.
Partner with us to benefit from:
- Custom R&D and Formulation: Our technical expertise ensures your patch meets exact therapeutic and safety requirements.
- High-Quality Manufacturing: We produce reliable, consistent patches you can trust for your patients.
- Scalable Bulk Production: We support healthcare distributors and brands with efficient, large-scale production.
Contact our experts today to discuss your custom transdermal patch development needs.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Prostate Pain Kidney Health Care Patch for Men
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What types of coughs can the far infrared cough relief patch address? Soothe Dry, Wet, and Persistent Coughs
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- How should missed doses of the Reliever Patch be handled? Safe Usage Guidelines
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How does the cough relief patch provide targeted relief? Direct, Soothing Comfort for Coughs & Chest Congestion














