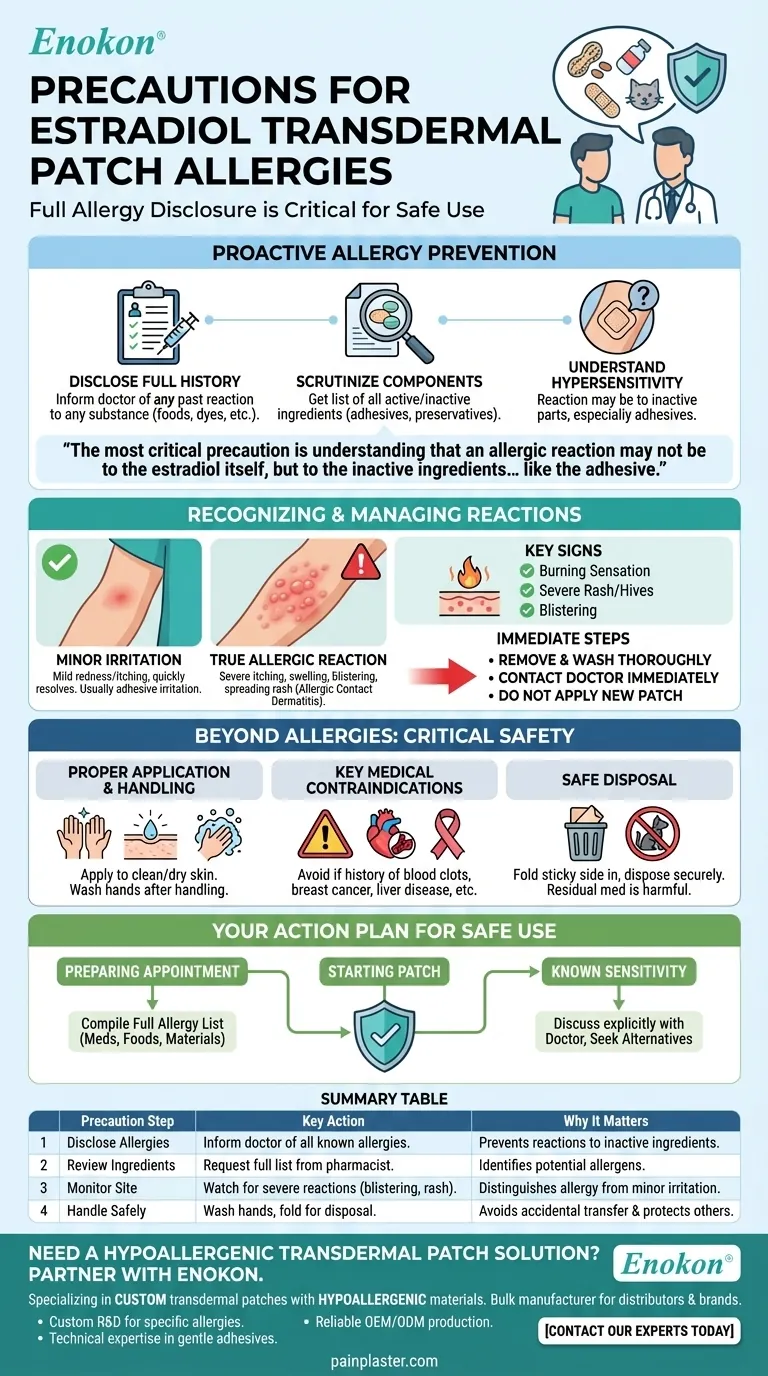
Before using any estradiol transdermal medication, you must inform your doctor about your complete allergic history. This includes not only reactions to estradiol or other medications but also any known allergies to foods, dyes, preservatives, or animals, as the patch contains multiple components beyond the active drug.
The most critical precaution is understanding that an allergic reaction may not be to the estradiol itself, but to the inactive ingredients in the transdermal patch, such as the adhesive. A thorough disclosure of all sensitivities to your healthcare provider is the foundation of safe use.
A Proactive Approach to Allergy Prevention
The key to avoiding an adverse reaction is comprehensive communication and careful product review before you ever apply the first patch. Your medical history provides the context your doctor needs to ensure the product is right for you.
Disclose Your Full Allergic History
Your doctor needs to know about any unusual or allergic reaction you have ever had to any substance.
A sensitivity to a specific dye used in a food product or a preservative in another medication could signal a potential reaction to an inactive ingredient within the estradiol patch.
Scrutinize the Product Components
While estradiol is a prescription product, the principle of reading the ingredients remains vital.
Ask your doctor or pharmacist for a list of all active and inactive ingredients in the specific brand of patch you are prescribed. The adhesives and other materials that make up the patch system are common culprits for skin reactions.
Understand Hypersensitivity
Hypersensitivity simply means you are allergic to one or more components in the product.
This could be the estradiol, but it is frequently an element of the patch delivery system. If you have a known sensitivity to medical adhesives, this is a critical piece of information to share.
Recognizing and Managing Skin Reactions
It is important to distinguish between minor, temporary irritation and a true allergic reaction, which requires immediate medical attention.
Differentiating Irritation from Allergy
Mild redness or itching at the application site that resolves quickly may be simple irritation from the adhesive.
A true allergic reaction, or allergic contact dermatitis, is more severe. It can involve intense itching, swelling, blistering, or a rash that spreads beyond the patch application site.
Key Signs of an Allergic Reaction
Watch for significant skin changes after applying a patch.
Symptoms like a burning sensation at the application site, a severe rash, or hives are clear indicators of an allergic response and should not be ignored.
Immediate Steps to Take
If you suspect an allergic reaction, gently remove the patch and wash the area thoroughly with soap and water.
Contact your doctor or pharmacist immediately for guidance. Do not apply a new patch until you have received medical advice.
Beyond Allergies: Critical Safety Precautions
Safe use of transdermal estradiol involves more than just allergy awareness. Proper handling and an understanding of major health contraindications are essential for your well-being.
Proper Application and Handling
Always apply the patch to a clean, dry, and unbroken area of skin as directed. Wash your hands thoroughly with soap and water after touching the patch to avoid transferring the medication to others.
Key Medical Contraindications
You should not use estradiol if you have a history of certain conditions.
These include a history of blood clots, breast cancer or other estrogen-dependent tumors, recent heart attack or stroke, liver disease, or unexplained vaginal bleeding.
Safe Disposal of Used Patches
A used patch still contains residual medication that can be harmful to others.
Fold the used patch in half so the adhesive side sticks to itself, and dispose of it in a location inaccessible to children or pets.
Your Action Plan for Safe Estradiol Use
Your approach should be tailored to where you are in your treatment journey. Proactive communication with your healthcare team is the single most important factor for safety.
- If you are preparing for your doctor's appointment: Compile a complete list of every known allergy or sensitivity you have, including reactions to medications, foods, and materials like adhesives.
- If you are about to start using the patch: Ask your pharmacist for the full ingredient list for your prescribed brand and familiarize yourself with the signs of an allergic reaction.
- If you have a known sensitivity to skin products or adhesives: Discuss this explicitly with your doctor, as they may be able to prescribe a different brand or formulation.
Ultimately, being an informed and active participant in your own healthcare is your greatest tool for ensuring safety and success with your treatment.
Summary Table:
| Precaution Step | Key Action | Why It Matters |
|---|---|---|
| Disclose Allergies | Inform your doctor of all known allergies (drugs, foods, adhesives). | Prevents reactions to inactive patch ingredients like adhesives or preservatives. |
| Review Ingredients | Request a full list of active/inactive components from your pharmacist. | Identifies potential allergens in the specific patch brand before use. |
| Monitor Application Site | Watch for severe itching, blistering, or rash beyond the patch area. | Distinguishes minor irritation from a true allergic reaction needing medical care. |
| Handle Safely | Wash hands after application; fold used patches for secure disposal. | Avoids accidental transfer of medication and protects children/pets. |
Need a Hypoallergenic Transdermal Patch Solution? Partner with Enokon.
If you or your patients experience sensitivities to patch adhesives or ingredients, Enokon specializes in developing custom transdermal patches with hypoallergenic materials. As a bulk manufacturer for healthcare distributors and brands, we offer:
- Custom R&D to address specific allergy concerns.
- Technical expertise in formulating gentle yet effective adhesives.
- Reliable, OEM/ODM-compliant production for pain plasters and hormone delivery systems.
Ensure patient safety and comfort—contact our experts today to discuss your custom patch requirements!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints














