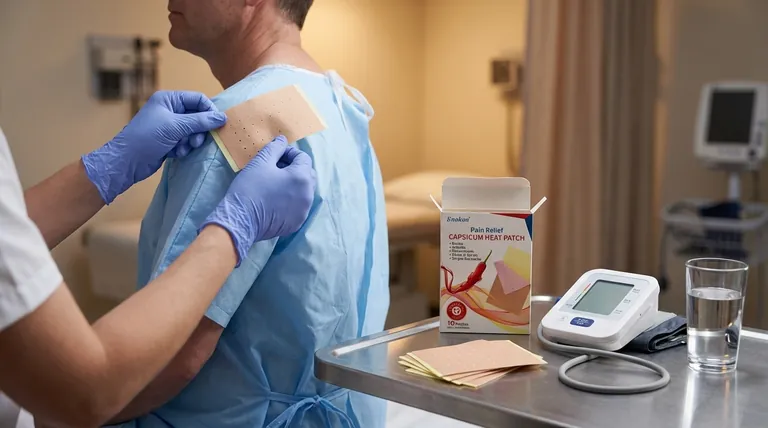
When using Capsaicin Transdermal Patch, several precautions must be taken to ensure safety and effectiveness. Key measures include avoiding application to sensitive areas like the face or scalp, wearing nitrile gloves during handling, and monitoring for blood pressure changes post-application. Patients with uncontrolled hypertension or cerebrovascular risks require special caution. Proper removal techniques (rolling inward) prevent aerosolization, and heat exposure should be minimized. Hypersensitivity to components must be ruled out before use, and accidental contact with untreated skin or mucous membranes should be avoided.
Key Points Explained:
-
Application Site Restrictions
- Never apply to the face, scalp, or mucous membranes due to risk of severe irritation.
- Avoid broken or irritated skin to prevent increased absorption and adverse effects.
-
Handling and Removal
- Wear nitrile gloves (not latex) during application to prevent unintended skin contact.
- Remove patches by gently rolling them inward to minimize aerosolization of capsaicin particles, which can cause respiratory or eye irritation.
-
Monitoring Post-Application
- Monitor blood pressure for at least 1 hour after application due to potential transient elevation.
- Patients with uncontrolled hypertension or cerebrovascular disease history need close supervision.
-
Heat Exposure
- Avoid direct heat (e.g., heating pads, hot showers) on treated areas, as it may intensify skin reactions or increase systemic absorption.
-
Hypersensitivity and Allergies
- Discontinue use if signs of allergy (rash, swelling) appear. Confirm absence of hypersensitivity to capsaicin or patch components beforehand.
-
Special Populations
- Use extreme caution in elderly patients or those with fragile skin.
- Keep patches out of reach of children—accidental exposure can cause severe discomfort.
-
Environmental Precautions
- Dispose of used patches in sealed containers to prevent secondary exposure.
- Wash hands thoroughly after glove removal, even if no direct contact occurred.
By adhering to these guidelines, users can mitigate risks while leveraging the therapeutic benefits of capsaicin for pain management. Have you considered how these protocols align with your facility’s safety standards for topical analgesics?
Summary Table:
| Precaution Category | Key Measures |
|---|---|
| Application Sites | Avoid face, scalp, mucous membranes, or broken skin. |
| Handling & Removal | Use nitrile gloves; roll patches inward to prevent aerosolization. |
| Post-Application | Monitor blood pressure for 1+ hour; high-risk patients need supervision. |
| Heat Exposure | Avoid direct heat (e.g., heating pads) to prevent intensified reactions. |
| Hypersensitivity | Discontinue if allergies occur; confirm tolerance beforehand. |
| Special Populations | Elderly/fragile skin: extreme caution; keep patches away from children. |
| Environmental Safety | Dispose in sealed containers; wash hands post-handling. |
Ensure safe, effective capsaicin transdermal therapy with Enokon’s expertise!
As a bulk manufacturer of high-quality transdermal patches and pain plasters, we help healthcare distributors and brands integrate these protocols seamlessly. Our technical team supports custom R&D for tailored solutions—contact us to discuss your needs or request samples.
Why Enokon?
✔ OEM/ODM capabilities for compliant formulations
✔ Rigorous safety testing aligned with global standards
✔ End-to-end support from development to logistics
Visual Guide

Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
















