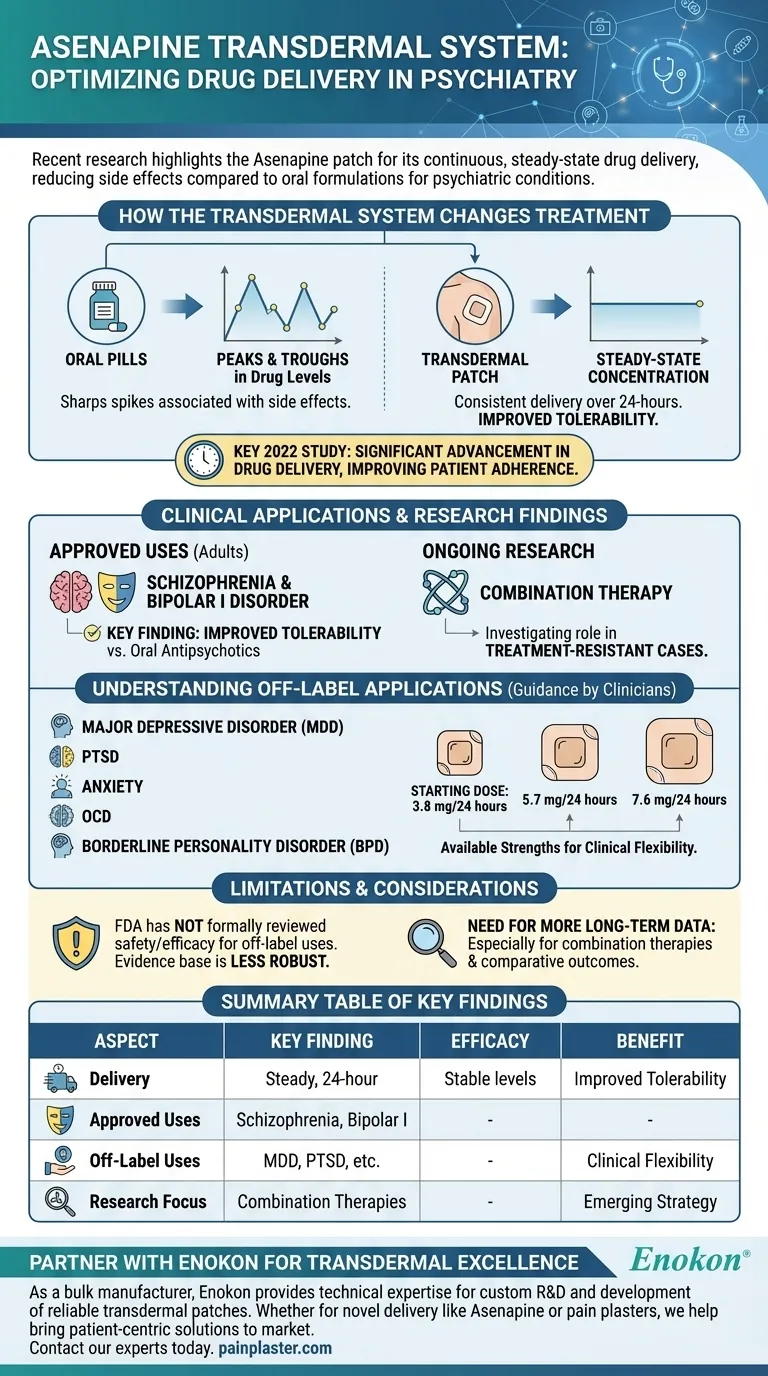
Recent research on the Asenapine transdermal system focuses on its efficacy in delivering a steady, continuous dose of medication for psychiatric conditions. A key 2022 study highlighted its potential to reduce side effects compared to oral formulations for schizophrenia and bipolar disorder, while ongoing investigations are exploring its use in combination therapies for treatment-resistant cases.
The core finding is that the Asenapine transdermal patch offers a significant advancement in drug delivery. By maintaining stable medication levels in the bloodstream, it aims to improve treatment tolerability and patient adherence for approved and potential off-label conditions.
How the Transdermal System Changes Treatment
A Novel Delivery Method
The Asenapine transdermal system is a patch applied to the skin. This method bypasses the digestive system, delivering the medication directly into the bloodstream over a 24-hour period.
Achieving Steady-State Concentration
Unlike oral pills that cause peaks and troughs in drug levels, the transdermal patch provides a steady release. This consistency is the primary mechanism behind its potential benefits.
The Impact on Side Effects
By avoiding the sharp spikes in concentration associated with oral dosing, the transdermal system may reduce the intensity or frequency of certain side effects. This was a central finding in recent clinical evaluations.
Clinical Applications and Research Findings
Approved Uses: Schizophrenia and Bipolar I
The system is primarily studied and approved for the treatment of schizophrenia in adults and for the manic or mixed episodes associated with bipolar I disorder.
Key Finding: Improved Tolerability
The 2022 study demonstrated that this steady delivery can lead to a more tolerable side effect profile. This is a critical advantage for patients who struggle with the adverse effects of oral antipsychotics.
Ongoing Research: Combination Therapy
Current research is actively investigating the patch's role in more complex scenarios. This includes its use alongside other medications for patients with treatment-resistant conditions.
Understanding Off-Label Applications
A Broad Spectrum of Potential Uses
Beyond its approved indications, clinicians have explored using the Asenapine transdermal system for a wide range of other conditions. These off-label uses include major depressive disorder (MDD), PTSD, anxiety, OCD, and borderline personality disorder (BPD).
Dosing and Strengths
For most off-label applications, the typical starting dose is 3.8 mg/24 hours. The patch is available in three strengths to allow for clinical flexibility: 3.8 mg/24 hours, 5.7 mg/24 hours, and 7.6 mg/24 hours.
Limitations and Considerations
The Distinction Between Approved and Off-Label
It is crucial to understand that off-label use means the FDA has not formally reviewed the drug's safety and efficacy for that specific condition. The evidence base for these uses is less robust than for its approved indications.
The Need for More Data
While initial findings are promising, more long-term data is needed. Research is still required to fully understand its efficacy in combination therapies and to compare its outcomes against other treatments comprehensively.
Making the Right Choice for Your Goal
- If your primary focus is treating schizophrenia or bipolar I: The transdermal system presents a valuable alternative, especially for patients sensitive to the side effects of oral medications.
- If your primary focus is exploring options for treatment-resistant conditions: Combination therapy involving the Asenapine patch is an area of active research, but it should be considered an emerging strategy.
- If your primary focus is an off-label condition like MDD or PTSD: This approach should be guided by an experienced clinician, typically starting at the lowest dose and proceeding with careful monitoring.
The Asenapine transdermal system represents a meaningful evolution in psychiatric pharmacology, shifting the focus toward optimizing drug delivery to improve patient outcomes.
Summary Table:
| Aspect | Key Finding |
|---|---|
| Efficacy | Steady, 24-hour delivery for stable medication levels. |
| Key Benefit | Improved tolerability and reduced side effects vs. oral formulations. |
| Approved Uses | Schizophrenia and bipolar I disorder in adults. |
| Off-Label Uses | MDD, PTSD, anxiety, OCD, BPD (starting dose: 3.8 mg/24h). |
| Research Focus | Combination therapies for treatment-resistant conditions. |
Ready to leverage the latest transdermal technology for your pharmaceutical brand?
As a bulk manufacturer of reliable transdermal patches, Enokon provides the technical expertise for custom R&D and development. Whether you are exploring novel drug delivery systems like the Asenapine patch or need a trusted partner for pain plasters, we can help you bring advanced, patient-centric solutions to market.
Contact our experts today to discuss your project and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














