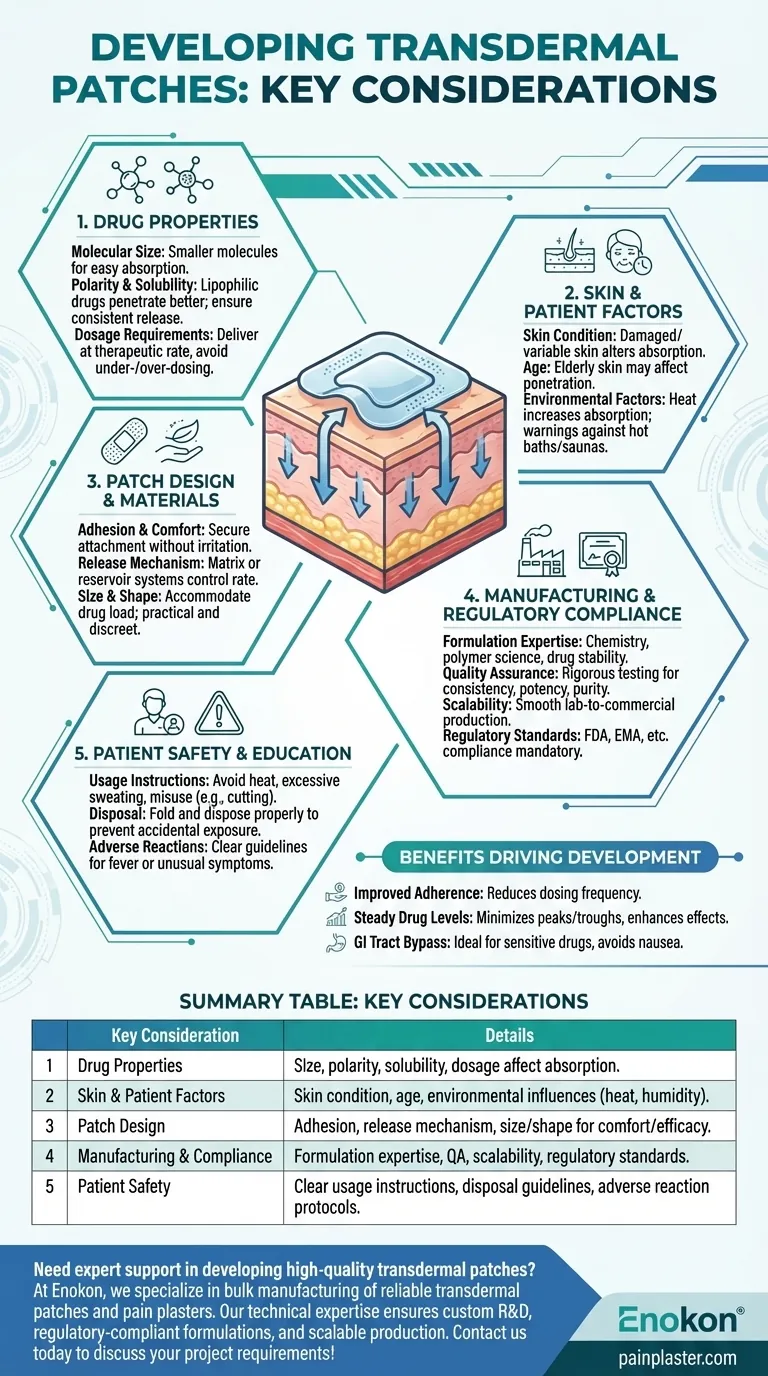
Developing transdermal patches requires careful consideration of multiple factors to ensure efficacy, safety, and patient compliance. Key aspects include drug properties (size, polarity, solubility), skin-related variables (condition, age), environmental influences (temperature, humidity), and manufacturing expertise (formulation, regulatory compliance). Additionally, patient education on proper usage and potential risks is critical. The design must balance drug delivery efficiency with comfort and practicality, while adhering to stringent quality and safety standards throughout development and production.

Key Points Explained:
-
Drug Properties
- Molecular Size: Smaller molecules are absorbed more easily through the skin, making them ideal candidates for transdermal patch delivery.
- Polarity & Solubility: Lipophilic (fat-soluble) drugs penetrate the skin more effectively than hydrophilic ones. The drug must also have adequate solubility to ensure consistent release.
- Dosage Requirements: The patch must deliver the drug at a therapeutic rate, avoiding under- or over-dosing.
-
Skin & Patient Factors
- Skin Condition: Damaged or highly variable skin (e.g., eczema, aging skin) can alter absorption rates.
- Age: Elderly patients may have thinner skin, affecting drug penetration.
- Environmental Factors: Temperature and humidity can influence drug release and skin permeability. For example, heat increases absorption, necessitating warnings against hot baths or saunas.
-
Patch Design & Materials
- Adhesion & Comfort: The patch must stay securely attached without causing irritation or discomfort.
- Release Mechanism: Matrix or reservoir systems control drug release rates. The choice depends on the drug’s properties and desired delivery profile.
- Size & Shape: Should accommodate the drug load while being practical for application (e.g., discreet, flexible).
-
Manufacturing & Regulatory Compliance
- Formulation Expertise: Requires knowledge of chemistry, polymer science, and drug stability.
- Quality Assurance: Rigorous testing ensures batch-to-batch consistency, potency, and purity.
- Scalability: Processes must transition smoothly from lab-scale feasibility studies to commercial production.
- Regulatory Standards: Compliance with FDA, EMA, or other regional guidelines is mandatory for approval.
-
Patient Safety & Education
- Usage Instructions: Patients must avoid heat exposure, excessive sweating, or misuse (e.g., cutting patches).
- Disposal: Used patches should be folded and disposed of properly to prevent accidental exposure.
- Adverse Reactions: Clear guidelines for handling fever or unusual symptoms (e.g., rapid drug release due to overheating).
-
Benefits Driving Development
- Improved Adherence: Reduces dosing frequency compared to oral or injectable routes.
- Steady Drug Levels: Minimizes peaks and troughs, enhancing therapeutic effects.
- GI Tract Bypass: Ideal for drugs degraded by stomach acid or causing nausea.
By addressing these factors holistically, developers can create effective, user-friendly transdermal patches that meet clinical and market needs. Would a patch’s adhesion performance in humid climates influence your choice of backing materials?
Summary Table:
| Key Consideration | Details |
|---|---|
| Drug Properties | Size, polarity, solubility, and dosage requirements affect absorption. |
| Skin & Patient Factors | Skin condition, age, and environmental influences (e.g., heat, humidity). |
| Patch Design | Adhesion, release mechanism, size/shape for comfort and efficacy. |
| Manufacturing & Compliance | Formulation expertise, quality assurance, scalability, regulatory standards. |
| Patient Safety | Clear usage instructions, disposal guidelines, and adverse reaction protocols. |
Need expert support in developing high-quality transdermal patches?
At Enokon, we specialize in bulk manufacturing of reliable transdermal patches and pain plasters for healthcare distributors and brands. Our technical expertise ensures custom R&D, regulatory-compliant formulations, and scalable production. Contact us today to discuss your project requirements!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use















