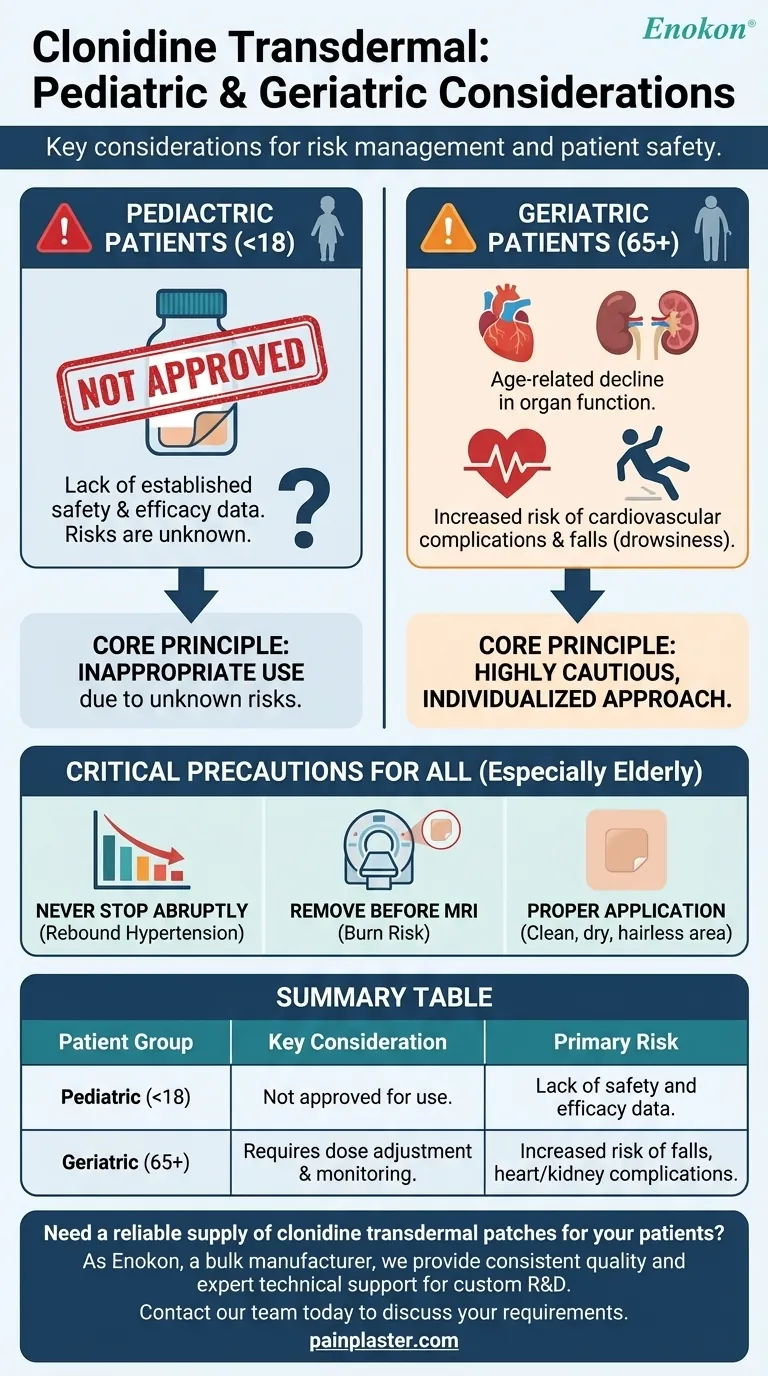
For pediatric patients, the primary consideration is straightforward: the clonidine transdermal system is not approved for use in anyone under 18 years of age. For geriatric patients, special consideration is required due to the increased likelihood of age-related heart and kidney problems, which can necessitate dose adjustments and heightened monitoring.
The core principle is risk management. For children, the risks are unknown due to a lack of safety and efficacy data, making its use inappropriate. For the elderly, the risks are known and magnified by age-related physiological changes, demanding a highly cautious and individualized approach.
The Pediatric Consideration: A Lack of Established Safety
The guidance regarding the use of clonidine transdermal patches in children is definitive and based on a crucial gap in medical research.
Not Approved for Use in Children
Regulatory bodies have not approved clonidine transdermal for use by any patient younger than 18 years old. This is a non-negotiable safety standard.
The Core Issue: Insufficient Data
The reason for this restriction is that appropriate studies to establish the safety and effectiveness of the clonidine patch in pediatric populations have not been performed. Without this data, the potential risks and benefits cannot be properly evaluated.
Geriatric Patients: A Focus on Physiological Changes
For older adults, the use of clonidine transdermal is not prohibited, but it requires careful management that accounts for the natural effects of aging on the body.
The Impact of Age-Related Organ Function
Many elderly patients experience a natural decline in kidney and heart function. Because clonidine affects the cardiovascular system and is cleared by the kidneys, reduced organ function can alter the drug's effects and lead to complications.
Increased Risk of Cardiovascular Complications
Older adults are more likely to have pre-existing conditions like sinus node dysfunction or AV block. Clonidine can worsen these heart rhythm disorders. A history of heart disease, coronary artery disease, or stroke also requires extremely careful monitoring.
Central Nervous System Effects and Fall Risk
Clonidine can cause drowsiness, dizziness, and impaired thinking or reaction times. In geriatric patients, these side effects significantly increase the risk of falls, which can lead to serious injury.
Critical Precautions for All Patients (Especially the Elderly)
While certain precautions apply to all users, they carry particular weight for older adults whose physiological reserves may be lower.
The Danger of Abrupt Discontinuation
Clonidine therapy should never be stopped suddenly. Doing so can cause a rapid and dangerous increase in blood pressure (rebound hypertension). The dose must be tapered down gradually under a doctor's supervision.
Managing Medical Procedures: MRI and Surgery
The patch contains aluminum in its backing. It must be removed before an MRI scan to prevent skin burns at the patch site. Physicians and dentists must always be informed about clonidine use before any surgery or procedure.
Proper Application and Skin Integrity
The patch should be applied to a clean, dry, and hairless area of the upper arm or chest. It's crucial to press it firmly to ensure it sticks. Avoid using lotions or oils on the area, as they can interfere with adhesion.
Making the Right Choice for Your Patient
Navigating the use of clonidine transdermal requires a clear understanding of the patient's specific physiological context.
- If your patient is a child (under 18): The clonidine transdermal system should not be used, as its safety and efficacy have not been established in this population.
- If your patient is an older adult: Assume a need for lower doses and heightened vigilance. Closely monitor for changes in heart function, kidney function, and cognitive effects like dizziness to prevent adverse outcomes.
- If your patient has any pre-existing heart or kidney condition: This warrants a thorough risk-benefit discussion with the prescribing physician and may necessitate more frequent follow-up appointments.
Ultimately, tailoring treatment to the individual's age and health status is the key to using this medication safely and effectively.
Summary Table:
| Patient Group | Key Consideration | Primary Risk |
|---|---|---|
| Pediatric (<18) | Not approved for use. | Lack of safety and efficacy data. |
| Geriatric (65+) | Requires dose adjustment & monitoring. | Increased risk of falls, heart/kidney complications. |
Need a reliable supply of clonidine transdermal patches for your patients? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors with consistent quality and expert technical support for custom R&D. Benefit from our expertise to ensure your patients receive safe and effective therapy. Contact our team today to discuss your requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














