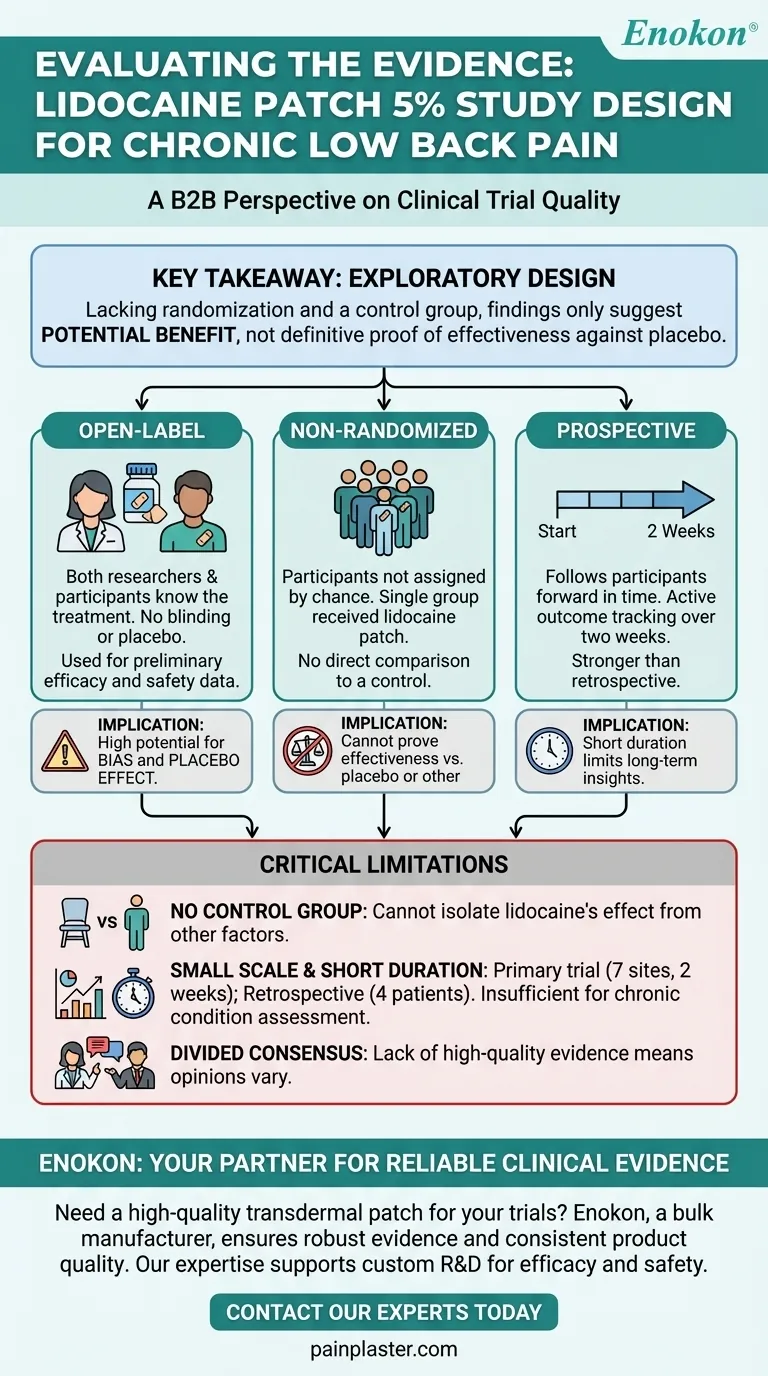
The primary study referenced was an open-label, non-randomized, prospective trial designed to evaluate the use of the lidocaine patch 5% for chronic low back pain. This preliminary study was supported by a separate retrospective case series involving a very small number of patients.
The key takeaway is that the studies used an early-stage, exploratory design. Because they lacked randomization and a control group, their findings can only suggest a potential for benefit, not definitively prove the patch's effectiveness compared to a placebo or other treatments.

Deconstructing the Study Design
To understand the quality of the evidence, we must break down the terminology used to describe the primary clinical trial. Each component reveals something important about the study's strengths and weaknesses.
What "Open-Label" Means
An open-label study is one where both the researchers and the participants know what treatment is being administered. There is no blinding or placebo.
This design is often used in early-phase trials to assess safety and gather preliminary data on efficacy without the complexity of a placebo control.
The Significance of "Non-Randomized"
In a non-randomized trial, participants are not assigned to treatment groups by chance. In this specific case, there was only one group; everyone received the lidocaine patch.
The lack of randomization prevents a direct, unbiased comparison between the treatment and a control, which is the gold standard for proving effectiveness.
Why "Prospective" Matters
A prospective study follows participants forward in time to gather new data. This is generally a stronger design than a retrospective study, which looks backward at existing records.
This study followed patients for two weeks, actively tracking their outcomes after applying the patch.
What This Design Means for the Evidence
The choice of an open-label, non-randomized design has significant implications for how we interpret the results. It signals that this research is exploratory, not conclusive.
High Potential for Bias
Without a control group or blinding, it's impossible to separate the true effect of the lidocaine from the placebo effect. Patients who believe a treatment will work often report feeling better, regardless of the treatment's active ingredients.
Furthermore, researchers' expectations can unintentionally influence how they assess patient outcomes.
A Starting Point, Not a Conclusion
The study itself concluded that adding the patch may be beneficial and that more rigorous trials were needed. This acknowledges the design's limitations.
Think of this type of study as a pilot program. It's designed to see if there's enough of a signal to justify investing in a larger, more expensive, and more definitive controlled trial.
Understanding the Key Limitations
It is critical to recognize the specific weaknesses inherent in this level of research. The evidence is considered limited because of several key factors.
The Absence of a Control Group
This is the most significant limitation. Without a group of similar patients receiving a placebo (a non-medicated patch), we cannot determine how much of the reported pain relief was due to the lidocaine itself versus other factors.
The Small Scale and Short Duration
The primary trial was conducted across seven sites for only two weeks. The supporting retrospective analysis only examined four patients.
Chronic conditions often require longer-term data to assess sustained efficacy and safety, which this study did not provide.
The Broader Scientific Consensus
Expert opinions remain divided precisely because of this lack of high-quality evidence. The consensus is that while some patients may find relief, the current research is insufficient to broadly recommend or oppose the use of lidocaine patches for chronic low back pain.
How to Apply This to Your Decision
Understanding the quality of this evidence is key to making an informed choice about potential treatments for chronic low back pain.
- If your primary focus is understanding the quality of this specific study: Its design is preliminary and serves only to generate a hypothesis, not to provide definitive proof of effectiveness.
- If your primary focus is evaluating treatment options for back pain: This study does not provide strong evidence to support the use of lidocaine patches over other established treatments or a placebo.
Ultimately, evaluating the source and quality of clinical evidence empowers you to have more productive conversations with your healthcare provider about your specific condition.
Summary Table:
| Study Design Component | Description | Implication for Evidence |
|---|---|---|
| Open-Label | No blinding; both researchers and patients know the treatment. | High potential for bias and placebo effect. |
| Non-Randomized | Single treatment group; no random assignment to control. | Cannot prove effectiveness vs. placebo or other treatments. |
| Prospective | Data collected forward in time over a 2-week period. | Stronger than retrospective, but short duration limits long-term insights. |
| Small Scale | Primary trial across 7 sites; retrospective analysis of 4 patients. | Limited generalizability of findings. |
Need a reliable, high-quality transdermal patch for your clinical trials or product line?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands, we understand the critical importance of robust evidence and consistent product quality. Our technical expertise supports custom R&D and development to ensure your patches meet the highest standards for efficacy and safety.
Let us help you develop a patch that stands up to rigorous clinical scrutiny. Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
People Also Ask
- Are lidocaine patches safe to use during pregnancy? A Guide to Making an Informed Choice
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief

















