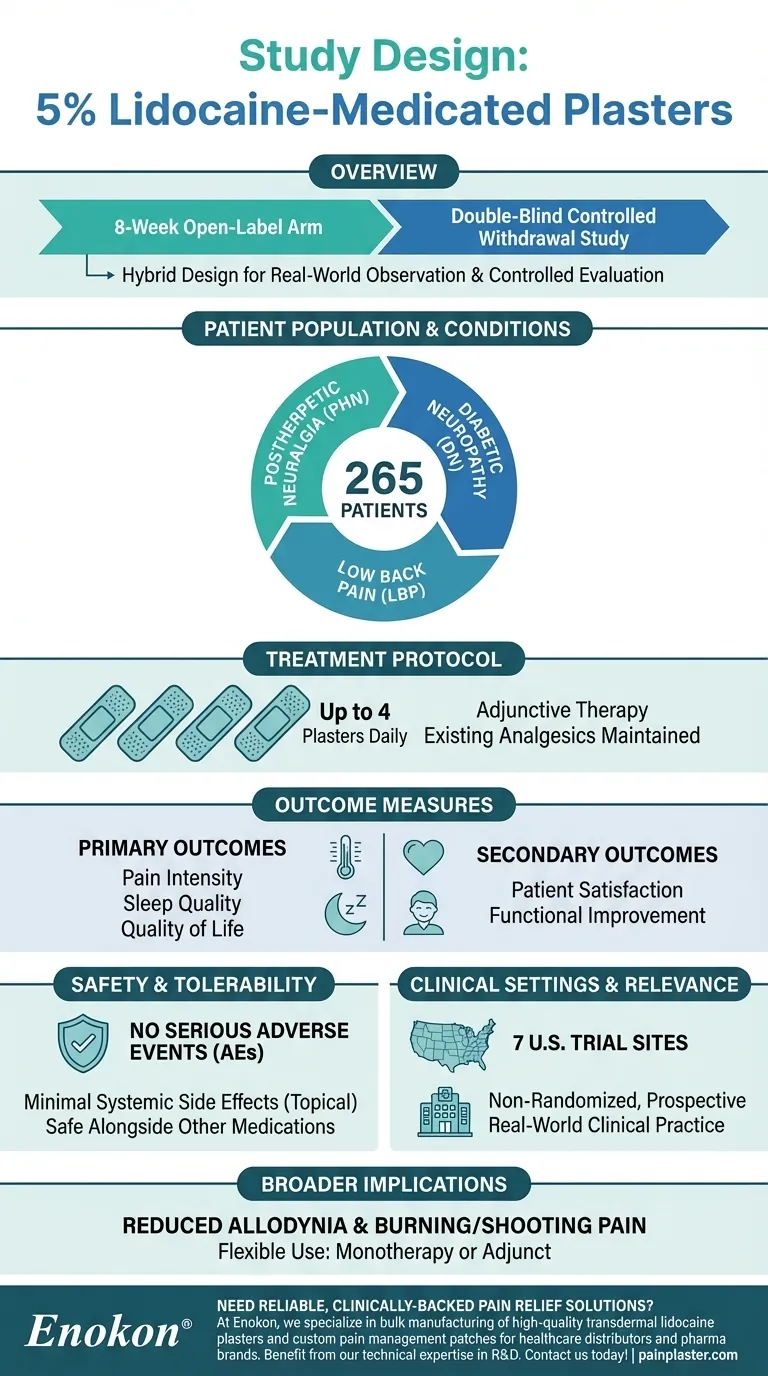
The study involving 5 percent lidocaine-medicated plasters was designed as an 8-week, open-label arm of a double-blind controlled withdrawal study. It aimed to evaluate the impact of these plasters on pain, sleep, quality of life, and patient satisfaction in individuals with postherpetic neuralgia (PHN) and other neuropathic pain conditions. Conducted across multiple clinical trial sites, the study allowed patients to apply up to four patches daily to areas of maximal pain while maintaining their existing analgesic regimens. The design emphasized real-world applicability by not randomizing participants and focusing on self-reported outcomes.

Key Points Explained:
-
Study Design and Duration
- The study was structured as an 8-week, open-label arm of a larger double-blind controlled withdrawal study.
- Open-label means both researchers and participants knew the treatment being administered, while the double-blind phase (not detailed here) would involve blinded comparisons.
- This hybrid design allowed for initial real-world observation (open-label) followed by controlled evaluation (double-blind).
-
Patient Population and Conditions
- Enrolled 265 patients with postherpetic neuralgia (PHN), a type of nerve pain following shingles.
- The plasters were also assessed for other neuropathic pain conditions, such as painful diabetic neuropathy (DN) and low back pain (LBP).
- Participants applied patches to areas of maximal pain, reflecting targeted therapy for localized neuropathic pain.
-
Treatment Protocol
- Patients used up to four lidocaine 5% plasters daily, changed every 24 hours.
- The plasters were adjunctive therapy, meaning patients continued their existing analgesic regimens without dose adjustments.
- This approach tested the plaster’s compatibility with other pain management strategies.
-
Outcome Measures
- Primary outcomes: Pain intensity, sleep quality, and quality of life.
- Secondary outcomes: Patient satisfaction and functional improvement.
- Data were self-reported, emphasizing patient-centric metrics.
-
Safety and Tolerability
- No serious adverse events (AEs) or drug interactions were reported.
- The plaster’s topical application minimized systemic side effects, supporting its use alongside other medications.
-
Clinical Settings and Real-World Relevance
- Conducted across 7 U.S. trial sites, including a large teaching hospital, to ensure diverse patient representation.
- The non-randomized, prospective design prioritized observational data, reflecting everyday clinical practice.
-
Broader Implications
- Demonstrated efficacy in reducing allodynia (pain from non-painful stimuli) and burning/shooting pain associated with PHN.
- Highlighted the plaster’s role as a monotherapy or adjunct, offering flexibility in pain management protocols.
By combining open-label observation with controlled evaluation, this study provided robust evidence for the plaster’s clinical utility in improving pain and quality of life for neuropathic pain patients.
Summary Table:
| Aspect | Details |
|---|---|
| Study Design | 8-week open-label arm of a double-blind controlled withdrawal study |
| Patient Population | 265 patients with PHN, diabetic neuropathy, or low back pain |
| Treatment Protocol | Up to four 5% lidocaine plasters daily, adjunctive to existing analgesics |
| Primary Outcomes | Pain intensity, sleep quality, quality of life |
| Secondary Outcomes | Patient satisfaction, functional improvement |
| Safety | No serious adverse events; minimal systemic side effects |
| Clinical Settings | 7 U.S. trial sites, non-randomized design for real-world relevance |
Need reliable, clinically-backed pain relief solutions for your patients?
At Enokon, we specialize in bulk manufacturing of high-quality transdermal lidocaine plasters and custom pain management patches. Our products are designed for healthcare distributors and pharma brands seeking evidence-based, patient-centric solutions. Benefit from our technical expertise in R&D to develop tailored formulations for neuropathic pain.
Contact us today to discuss partnership opportunities or request samples!
Visual Guide
Related Products
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- What are the key components of a pain relief patch? Unlock the Science of Targeted Pain Relief
- What are the benefits of Pain Relief Patch being licensed as a medicine? Guaranteed Efficacy & Safety
- How does Pain Relief Patch deliver its active ingredients? | Targeted Relief Explained
- Can the pain relief patch be used with oral pain relief products? Avoid Dangerous Drug Interactions
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide















