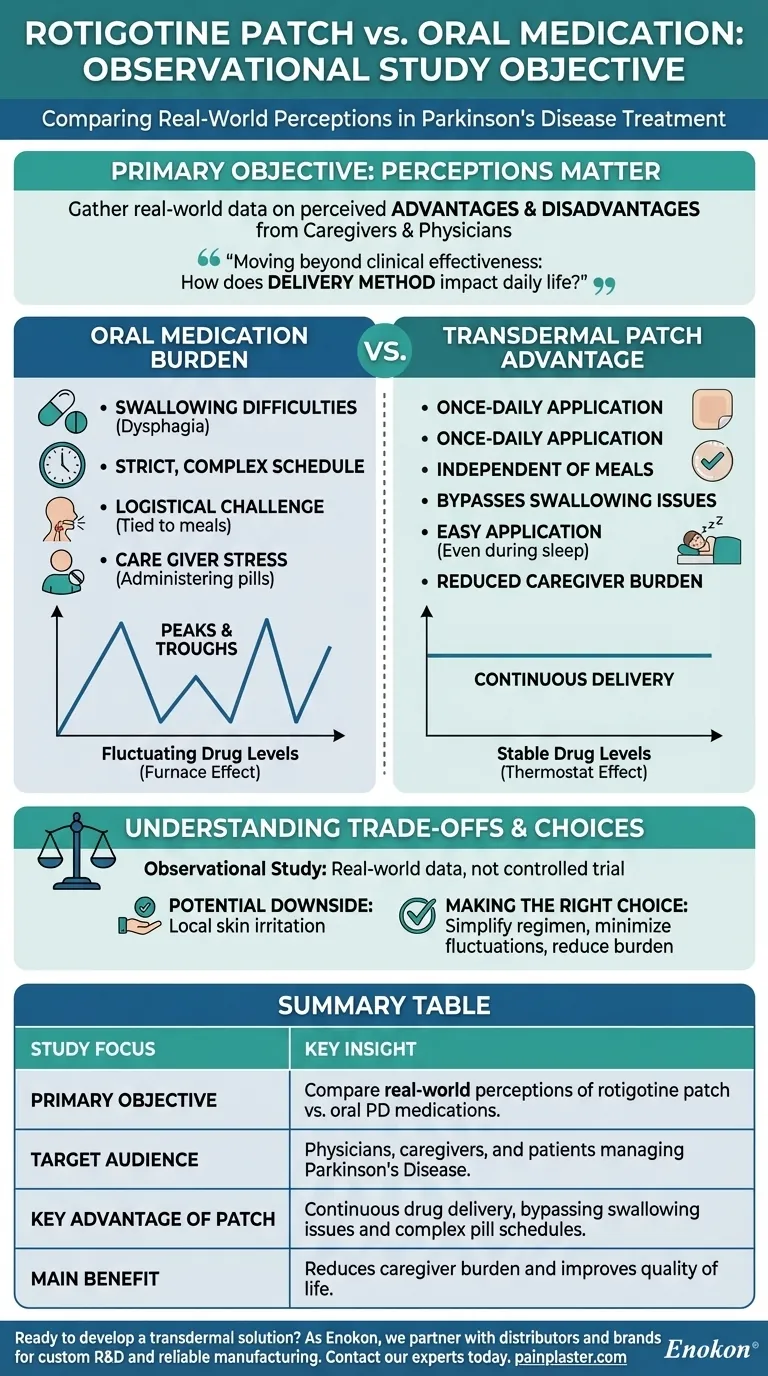
The primary objective of the observational study was to gather real-world data on how caregivers and physicians perceive the advantages and disadvantages of the rotigotine transdermal patch when compared to traditional oral medications for Parkinson's Disease (PD).
This study moved beyond simple clinical effectiveness to ask a more practical question: How does the method of delivering medication—a skin patch versus a pill—impact the daily lives of patients and the people who care for them?

Why Perceptions Matter More Than You Think
To understand the goal of this study, you must first appreciate the daily challenges of managing Parkinson's Disease. The choice of medication delivery is not just a technical detail; it has profound, real-world consequences for patients, caregivers, and physicians.
The Burden of Oral Medication
For many patients with Parkinson's, oral medication presents significant hurdles. As the disease progresses, swallowing difficulties (dysphagia) can make taking pills a distressing or even dangerous task.
Furthermore, oral medications often require a strict schedule, sometimes multiple times a day. This creates a constant logistical challenge, tying the patient's routine to a pill bottle and often requiring careful timing around meals.
The Caregiver's Critical Role
Caring for someone with PD is a demanding responsibility. The caregiver is often in charge of ensuring medications are taken correctly and on time.
This study specifically sought caregiver input because they directly experience the effort involved. Administering pills to a patient who has trouble swallowing, is asleep, or is experiencing nausea can be a major source of stress and caregiver burden.
The Physician's Viewpoint
From a physician's perspective, the ideal treatment is one the patient can adhere to consistently. They must consider factors like ease of use, the reliability of the dose, and how simple it is to monitor and adjust treatment.
A complex oral regimen can lead to missed doses, which in turn leads to less effective symptom control.
How a Transdermal Patch Changes the Dynamic
The rotigotine transdermal patch was developed to address the core challenges of oral delivery by leveraging a different biological mechanism.
The Principle of Continuous Delivery
Oral medications create peaks and troughs in the drug levels in your bloodstream. A transdermal patch, by contrast, provides continuous dopaminergic stimulation by releasing the medication slowly and steadily through the skin.
Think of it like heating a room. Oral medication is like a furnace that blasts on and off, creating temperature swings. A patch is like a modern thermostat that maintains a constant, stable temperature.
Practical Advantages in Daily Life
This steady delivery translates into several practical benefits that the study aimed to quantify through perception ratings:
- Once-daily application: Replaces multiple daily pills with a single patch.
- Independence from meals: The patch works regardless of when or what the patient eats.
- Bypasses swallowing issues: A crucial benefit for patients with dysphagia.
- Application to sleeping patients: Caregivers can apply a new patch without waking the patient.
Understanding the Trade-offs
No treatment method is perfect, and this study's design—rating both advantages and disadvantages—was intended to capture a balanced view. The goal was to understand the real-world net benefit as perceived by users.
Observational vs. Experimental
It's important to note this was a non-interventional, cross-sectional study. This means it observed routine clinical practice as it was, without controlling variables. Its strength is capturing "real-world" data, but it cannot prove cause-and-effect with the same certainty as a randomized controlled trial.
Potential Downsides of Patches
While the references focus on the study's objective, any transdermal system has potential trade-offs. The most common issue with patches is local skin irritation at the application site. A physician must weigh this against the significant benefits of bypassing the gastrointestinal system.
Making the Right Choice for Your Goal
The insights from a study like this are designed to help tailor treatment to an individual's specific circumstances.
- If your primary focus is simplifying the daily medication regimen: The patch provides a clear advantage with its once-daily application that is not tied to meal schedules.
- If the patient experiences significant swallowing difficulties: A transdermal patch is a fundamentally better delivery system, as it completely avoids the need to swallow medication.
- If your goal is to minimize symptom fluctuations: The patch's continuous delivery is designed to provide more stable drug levels, which may help reduce "on-off" periods associated with oral levodopa.
- If reducing caregiver burden is a key priority: The ease of applying a patch, even to a sleeping patient, can significantly lighten the load on caregivers.
Ultimately, this study's objective was to provide the data needed to see treatment as a holistic decision, balancing clinical efficacy with quality of life.
Summary Table:
| Study Focus | Key Insight |
|---|---|
| Primary Objective | Compare real-world perceptions of rotigotine patch vs. oral PD medications. |
| Target Audience | Physicians, caregivers, and patients managing Parkinson's Disease. |
| Key Advantage of Patch | Continuous drug delivery, bypassing swallowing issues and complex pill schedules. |
| Main Benefit | Reduces caregiver burden and improves quality of life through simplified, once-daily application. |
Ready to develop a transdermal solution that improves patient adherence and quality of life?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharma distributors and brands. Our technical expertise ensures custom R&D and development to create effective, user-friendly patch systems tailored to your specific therapeutic needs.
Contact our experts today to discuss how we can support your next project with our proven manufacturing capabilities and innovation.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints













