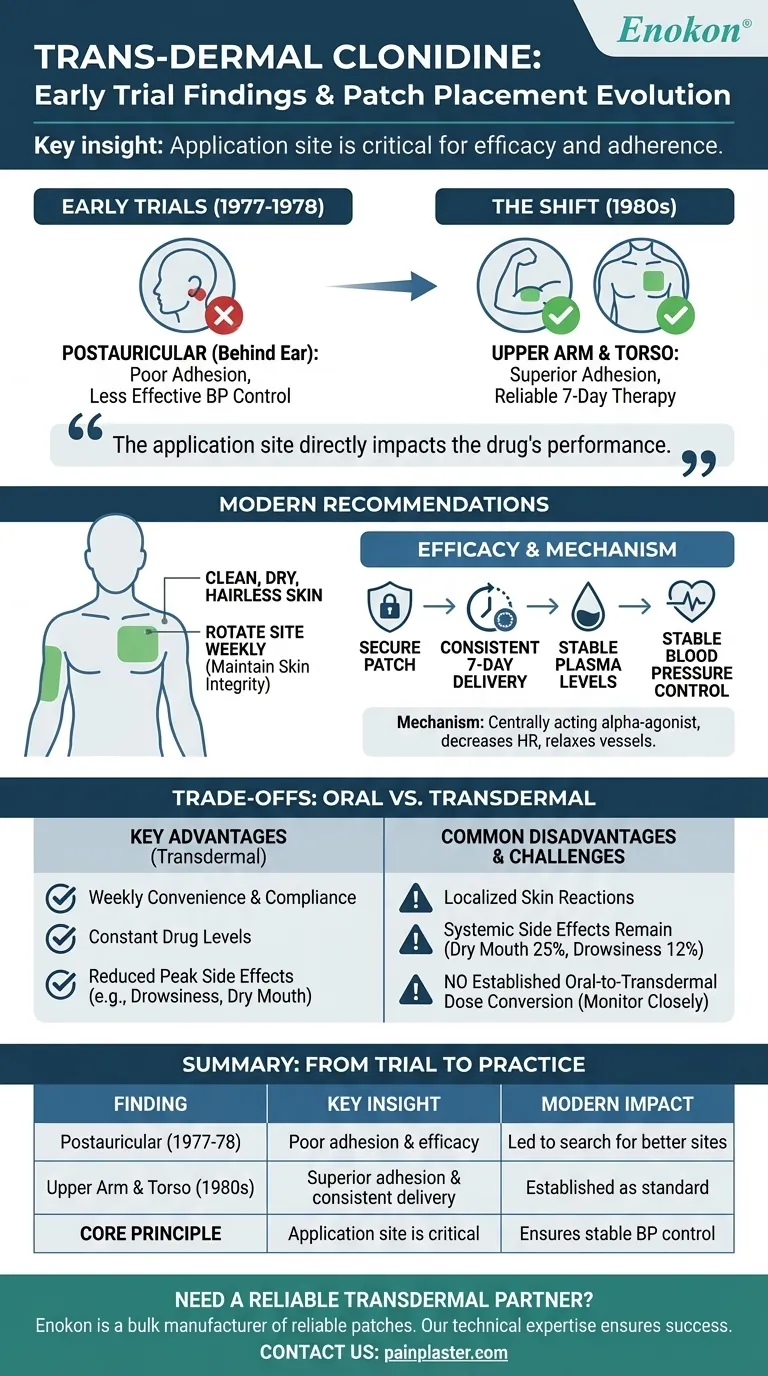
To be clear, early clinical trials of transdermal clonidine found that patch placement is critical for both adherence and efficacy. Initial trials in 1977-1978 tested the area behind the ear (postauricular) and discovered it resulted in poor patch adhesion and less effective blood pressure control. Subsequent studies in the 1980s determined that applying a larger patch to the upper arm provided much more reliable and effective results.
The core finding from early research is that the application site directly impacts the drug's performance. This established the upper arm and torso as the standard locations to ensure the patch remains secure and delivers a consistent, therapeutic dose for the full seven days.
The Evolution of Patch Placement: From Trial to Practice
Understanding the history of clonidine patch placement clarifies why current recommendations are so specific. The initial hypothesis about an ideal location did not hold up to real-world clinical data.
Early Trials and Postauricular Placement
The first trials in the late 1970s explored the postauricular area (behind the ear) as a potential application site.
Researchers quickly found this location was suboptimal. Patients experienced poor patch adherence, and the resulting blood pressure control was less effective than desired.
The Shift to the Upper Arm and Torso
Following the initial findings, researchers in the 1980s began testing other locations.
These later studies demonstrated that the upper arm or upper chest were far superior sites. They provided a stable, flat surface that improved adhesion and, consequently, therapeutic outcomes.
The Modern Recommendation for Application
Today's clinical guidelines are a direct result of this early research.
The patch should be applied to a clean, dry, and hairless area of skin on the upper outer arm or upper chest. It is crucial to rotate the application site each week to maintain skin integrity.
Why Application Site Matters for Efficacy
The location of the patch is not arbitrary; it is foundational to how transdermal clonidine works. The goal is to create a steady, continuous release of medication into the bloodstream.
Ensuring Consistent Drug Delivery
Transdermal clonidine is designed to deliver a constant dose (e.g., 0.1, 0.2, or 0.3 mg) per day over a seven-day period.
A secure patch on a stable surface ensures this consistent delivery, which is essential for maintaining stable blood pressure. Poor adherence leads to fluctuating drug levels and poor clinical control.
The Mechanism of Action
Clonidine is a centrally acting alpha-agonist hypotensive agent.
It works by decreasing heart rate and relaxing blood vessels, which allows blood to flow more easily. This mechanism requires steady plasma concentrations to be effective without causing sharp drops or peaks in blood pressure.
Preparing the Skin for Optimal Adherence
Proper site preparation is as important as site selection.
Avoid skin with wrinkles, folds, scars, cuts, or any irritation. The area should not be recently shaved, as this can increase irritation and affect drug absorption.
Understanding the Trade-offs of Transdermal Delivery
While effective, the transdermal patch has a distinct profile of advantages and disadvantages compared to oral clonidine.
Key Advantages
The primary benefit is convenience and compliance, with therapy lasting a full week after a single application.
This delivery system maintains constant drug levels, which can reduce the frequency of certain side effects like drowsiness and dry mouth that are more common with the peaks of oral dosing.
Common Disadvantages and Side Effects
The most significant disadvantage is the potential for localized skin reactions at the application site.
Systemic side effects can still occur, including dry mouth (25%), drowsiness (12%), fatigue (6%), and headache (5%). The patch is also typically more expensive than oral tablets.
Dosing and Titration Challenges
A critical point of caution is that there is no established oral-to-transdermal dose conversion.
When switching a patient from oral to transdermal clonidine, their blood pressure must be monitored closely to ensure the dose is titrated correctly.
Making the Right Choice for Your Goal
Applying the clonidine patch correctly is essential for achieving the desired therapeutic outcome. Your clinical goal should guide your patient counseling and management.
- If your primary focus is maximizing efficacy: Emphasize the strict adherence to approved application sites on the upper arm or torso and the importance of proper skin preparation before application.
- If your primary focus is patient adherence: Highlight the convenience of a weekly patch but stress that rotating the site each week is mandatory to prevent the skin irritation that can lead to discontinuation.
- If your primary focus is managing side effects: Counsel that while the patch may reduce some systemic effects, local skin reactions are common and systemic symptoms like dry mouth still require monitoring.
Ultimately, understanding the clinical rationale behind patch placement empowers you to achieve better blood pressure control for your patients.
Summary Table:
| Early Trial Finding | Key Insight | Impact on Modern Use |
|---|---|---|
| Postauricular (behind ear) site (1977-78) | Poor patch adhesion and efficacy | Led to search for better sites |
| Upper Arm & Torso site (1980s) | Superior adhesion and consistent drug delivery | Established as the standard for reliable 7-day therapy |
| Core Principle | Application site is critical for consistent plasma levels | Ensures stable blood pressure control |
Need a reliable transdermal patch partner?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your product's success, from custom R&D to development, helping you avoid the pitfalls of poor adhesion and inconsistent delivery highlighted in historical trials.
Contact our experts today to discuss your transdermal project and benefit from our proven development process.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief

















