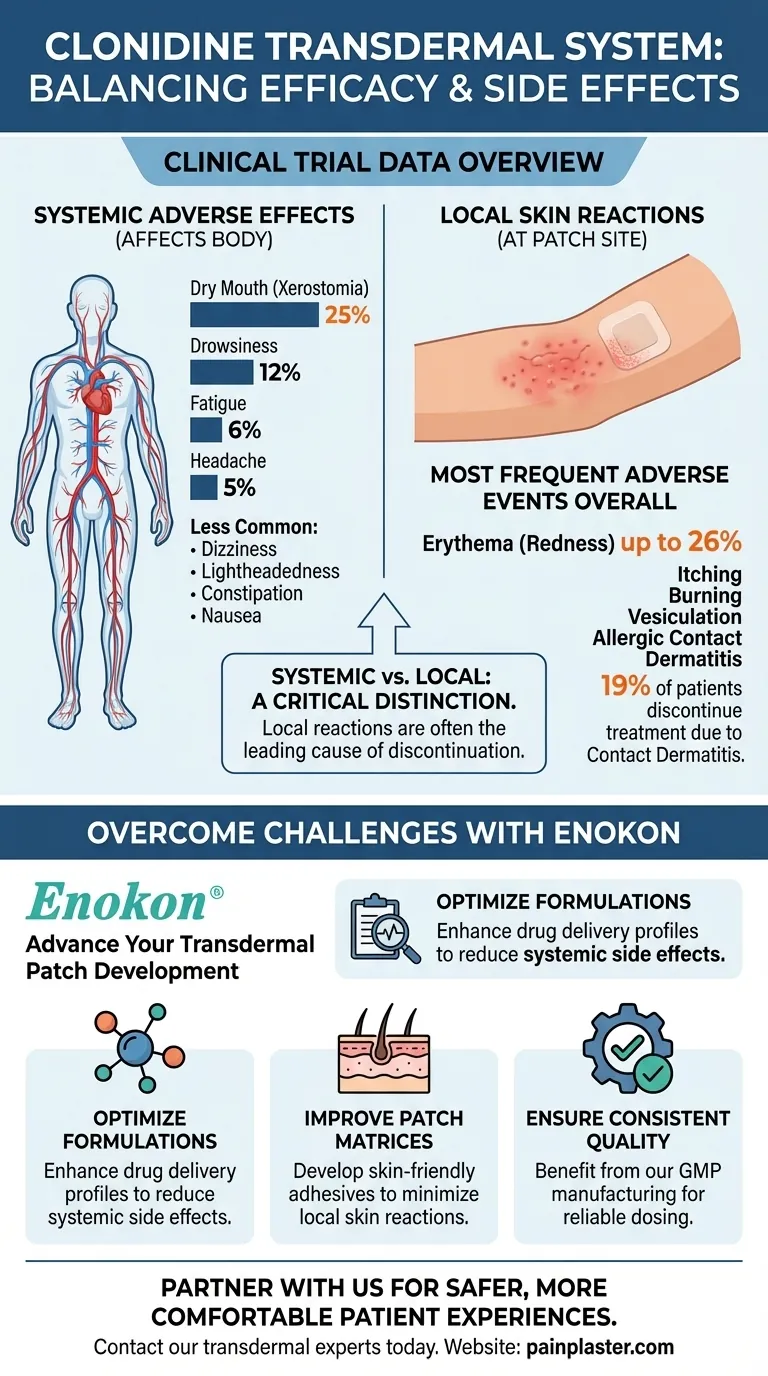
Based on clinical trial data, the most common systemic adverse effects observed with the clonidine transdermal system are dry mouth, occurring in 25% of patients, and drowsiness, occurring in 12% of patients. Other frequently reported systemic effects include fatigue (6%) and headache (5%).
While systemic effects like dry mouth and drowsiness are notable, it is critical to understand that local skin reactions at the patch site are often the most frequent adverse events overall, leading a significant number of patients to discontinue use.
A Deeper Look at Systemic Effects
Systemic effects occur when a drug is absorbed through the skin into the bloodstream, allowing it to circulate and affect the entire body. The clonidine patch is designed to do exactly this, providing a steady dose over several days.
The Most Prevalent Reactions
The two dominant systemic side effects are dry mouth (xerostomia) and drowsiness. These occur because clonidine acts on alpha-2 adrenergic receptors in the central nervous system, which helps lower blood pressure but also reduces saliva production and can cause sedation.
Other Common Systemic Complaints
Beyond the most common issues, a range of other systemic effects were reported, though at lower frequencies. These include fatigue, headache, dizziness, and lightheadedness, especially when standing up.
Less Frequent Systemic Issues
Patients have also reported lethargy, sedation, insomnia, constipation, nausea, changes in taste, and sexual dysfunction. These effects are considered less common but are important to be aware of when evaluating the medication.
The Critical Distinction: Systemic vs. Local Reactions
A comprehensive understanding requires differentiating between the drug's effects on the body (systemic) and its effects on the skin where the patch is applied (local).
Why Local Reactions Are So Common
The most frequent adverse reactions reported with the clonidine patch are dermatological. Data shows localized skin reactions like erythema (redness) can affect up to 26% of patients.
Other common local issues include itching, burning, vesiculation (blistering), and allergic contact dermatitis, which can be caused by the clonidine itself or the adhesives and other components in the patch.
The Impact of Local Reactions
These skin-related issues are not trivial. Studies indicate that allergic contact sensitization occurs in about 5% of patients, and approximately 19% of all patients discontinue treatment specifically because of contact dermatitis.
Understanding the Trade-offs
The use of a transdermal system like the clonidine patch involves a specific set of benefits and drawbacks compared to oral medication.
The Benefit: Stable Drug Delivery
The primary advantage of a patch is that it delivers a consistent amount of medication into the bloodstream over several days. This avoids the peaks and troughs in drug levels associated with taking pills, which can sometimes help moderate side effects.
The Inherent Drawback: The Skin Barrier
The skin is a natural barrier, and forcing a medication through it can cause irritation. This is a fundamental challenge for all transdermal drug delivery systems, not just clonidine.
Potential for Allergic Response
Beyond simple irritation, some individuals may develop a true allergic reaction to the drug or patch components. Though less common, reports have included maculopapular rash, urticaria (hives), and angioedema (swelling).
Making an Informed Decision
Choosing the right treatment involves weighing the potential for both systemic and local side effects against your specific health profile and goals.
- If your primary focus is treatment efficacy: The clonidine patch offers a stable delivery method, but you must be prepared to monitor for drowsiness and significant dry mouth.
- If you have sensitive skin or a history of skin allergies: Be aware that local skin reactions are the most common adverse event and the leading reason for discontinuing the patch.
- If you are balancing convenience and side effects: The key is to communicate proactively with your healthcare provider about any reaction, whether it's systemic (like fatigue) or local (like itching or redness at the patch site).
Understanding the complete side effect profile of the clonidine patch empowers you and your provider to manage your treatment effectively.
Summary Table:
| Systemic Adverse Effect | Approximate Frequency in Clinical Trials |
|---|---|
| Dry Mouth (Xerostomia) | 25% of patients |
| Drowsiness | 12% of patients |
| Fatigue | 6% of patients |
| Headache | 5% of patients |
| Dizziness / Lightheadedness | Less Common |
Develop Your Own Advanced Transdermal Patch with Fewer Side Effects
Navigating the balance between drug efficacy and patient tolerance is a core challenge in transdermal delivery. At Enokon, we specialize in helping healthcare and pharmaceutical brands overcome these hurdles.
As a bulk manufacturer of reliable transdermal patches and pain plasters, our technical expertise is your advantage. We offer custom R&D and development services to:
- Optimize Formulations: Enhance drug delivery profiles to potentially reduce systemic side effects like drowsiness and dry mouth.
- Improve Patch Matrices: Develop skin-friendly adhesives and components to minimize the local skin reactions that often lead to treatment discontinuation.
- Ensure Consistent Quality: Benefit from our GMP manufacturing for patches that deliver stable, reliable dosing.
Partner with us to create a safer, more comfortable patient experience. Let's discuss your project requirements.
Contact our transdermal experts today for a consultation on custom patch development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints















