To be clear, the majority of patients using the fentanyl patch experience no skin irritation at all. When skin reactions do occur, they are typically localized and affect a small percentage of users, with the most common issues being itching, swelling, and the formation of small bumps or pustules at the application site.
While localized skin reactions to the fentanyl patch are generally mild and uncommon, they are part of a much broader spectrum of potential side effects. The primary risks of this medication are systemic and severe, demanding strict adherence to medical guidance to ensure safety.
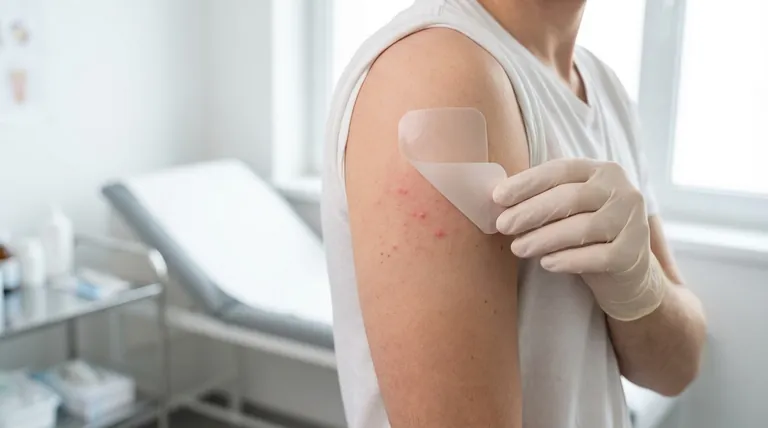
The Full Spectrum of Fentanyl Side Effects
Understanding the potential reactions involves looking beyond the skin to the medication's powerful effects on the entire body.
Localized Skin Reactions
The most commonly reported skin reactions occur in 2% to 8% of patients.
These reactions are confined to the patch application area and may include itching, edema (swelling), papules (small raised bumps), and pustules (pus-filled bumps). Mild redness or irritation at the site is also possible.
Common Systemic Side Effects
Many users experience systemic side effects, which are related to the drug's effects on the central nervous system.
These often include nausea, vomiting, constipation, drowsiness, dizziness, or headache. For some individuals, these effects may lessen as their body adapts to the medication over time.
Serious Medical Complications
Fentanyl is a potent opioid with risks that require immediate medical attention if they appear.
Serious side effects can include severe respiratory depression (dangerously slowed breathing), especially when starting the patch or increasing the dose. Other critical risks are low blood pressure (dizziness, fainting), adrenal insufficiency (fatigue, weakness), and serotonin syndrome (shivering, fever, seizures).
Critical Risks and Misuse Dangers
The most severe outcomes associated with the fentanyl patch are almost always linked to improper use, interactions, or misunderstanding its potency.
Risk of Overdose and Addiction
Fentanyl carries a high risk for abuse and addiction, which can lead to overdose and death.
Using the patch with alcohol or other central nervous system depressants dramatically increases the risk of fatal breathing problems.
Improper Handling and Application
A fentanyl patch must never be cut or damaged. Doing so can cause the medication to release too quickly, delivering a dangerous and potentially fatal dose all at once.
Similarly, changing patches more frequently than the prescribed three-day interval can lead to a toxic buildup of the drug in your system.
Potential for Drug Interactions
Fentanyl's effects can be dangerously altered by other medications.
Products known to interact include certain pain medications, naltrexone, and especially MAO inhibitors, which can cause a life-threatening drug interaction. Always inform your healthcare provider of all medications you are taking.
Making the Right Choice for Your Health
Navigating treatment with a fentanyl patch requires a clear understanding of the balance between its benefits for severe pain and its significant risks.
- If your primary concern is skin irritation: Be aware that this is uncommon and usually mild, but report any persistent or worsening reactions to your doctor.
- If your primary concern is overall safety: Adhere strictly to your doctor's instructions on dosage, timing, and application, and never alter the patch in any way.
- If you are taking other medications: Ensure your doctor and pharmacist have a complete list of everything you take to prevent dangerous drug interactions.
Ultimately, safe and effective use of the fentanyl patch depends on constant communication with your healthcare provider and an unwavering commitment to following their guidance precisely.
Summary Table:
| Type of Reaction | Common Symptoms | Key Notes |
|---|---|---|
| Localized Skin | Itching, swelling, redness, bumps/pustules | Affects 2-8% of users; usually mild and confined to application site. |
| Common Systemic | Nausea, vomiting, constipation, drowsiness, dizziness | Related to drug's effect on CNS; may lessen over time. |
| Serious Systemic | Severe respiratory depression, low blood pressure, serotonin syndrome | Require immediate medical attention; high risk of overdose and addiction. |
Need a reliable, high-quality transdermal patch for your healthcare brand or distribution network?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We understand the critical importance of safety, consistency, and precise drug delivery. Our technical expertise ensures custom R&D and development to meet your specific formulation needs, helping you bring safe and effective products to market.
Let's discuss your project requirements. Contact our experts today to learn how we can support your product development.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained


















