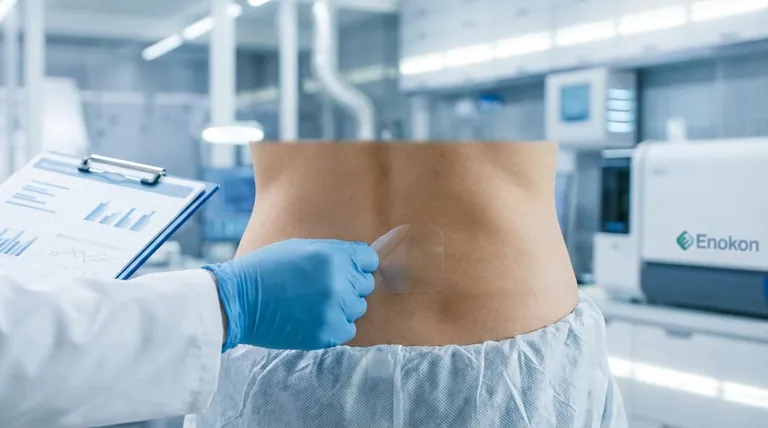
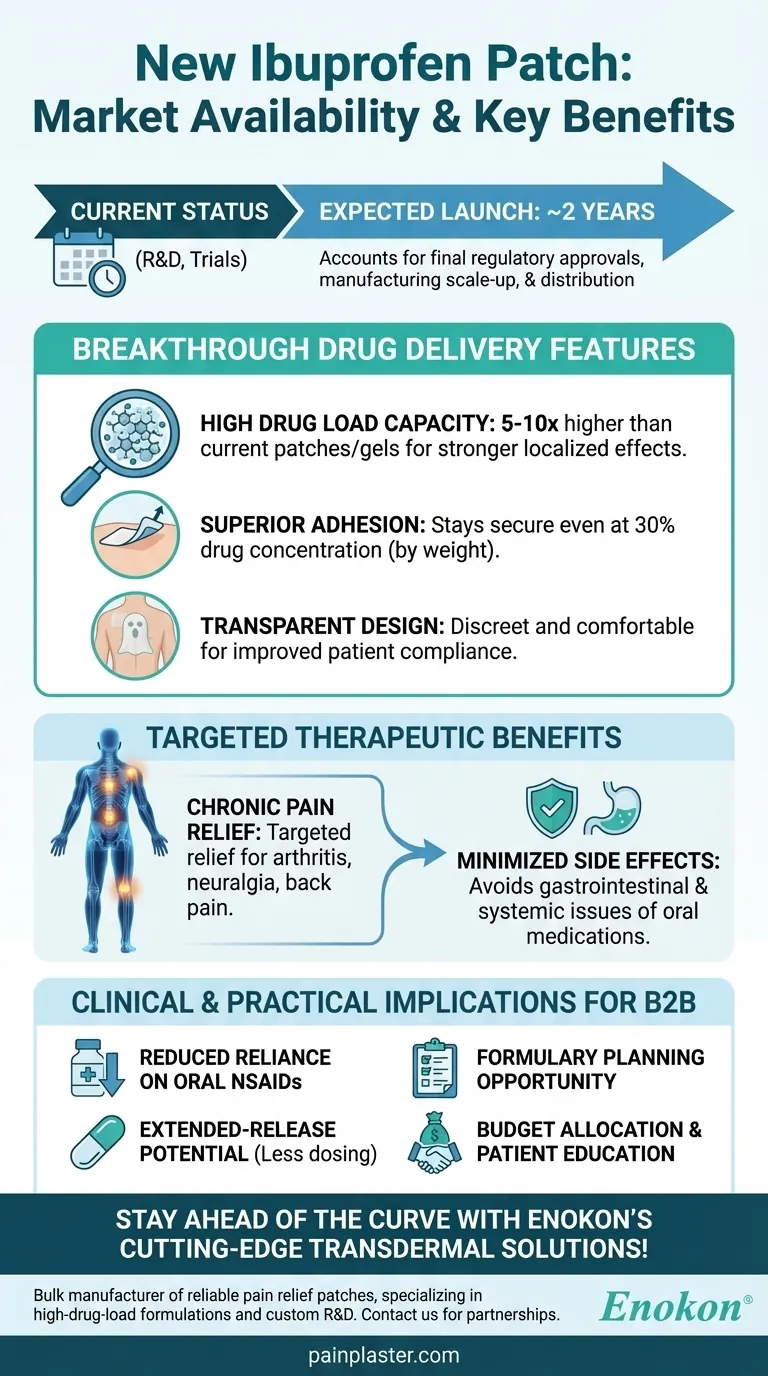
The new ibuprofen patch, a promising advancement in transdermal drug delivery, is expected to reach the market in approximately two years. This innovative bup patch offers significant advantages over current treatments, including a drug load 5-10 times higher than existing medical patches and gels, while maintaining excellent skin adhesion even at concentrations up to 30% of the patch weight. Its transparent design and ability to deliver targeted relief for conditions like back pain, neuralgia, and arthritis without the systemic side effects of oral medications make it a highly anticipated product in pain management.
Key Points Explained:
-
Market Availability Timeline
- The patch is projected to launch in about 2 years, based on consistent references to this timeline.
- This timeframe likely accounts for final regulatory approvals, manufacturing scale-up, and distribution logistics.
-
Breakthrough Drug Delivery Features
- High Drug Load Capacity: The patch can carry 5-10 times more ibuprofen than current gels or patches, enabling stronger localized effects.
- Superior Adhesion: Even at 30% drug concentration (by weight), the patch remains securely attached to the skin, addressing a common limitation of transdermal systems.
- Transparent Design: Improves patient compliance by being discreet and comfortable for daily wear.
-
Targeted Therapeutic Benefits
- Designed for chronic pain conditions (e.g., arthritis, neuralgia) where sustained relief is needed.
- Avoids gastrointestinal and systemic side effects associated with oral ibuprofen by delivering the drug directly through the skin.
-
Clinical and Practical Implications
- Could reduce reliance on oral NSAIDs, benefiting patients with sensitivities to high-dose pills.
- The extended-release potential may decrease dosing frequency compared to topical gels requiring reapplication.
-
Why This Timeline Matters for Purchasers
- Healthcare providers and procurement teams should monitor updates for formulary planning.
- Early awareness allows for budget allocation and patient education about this non-invasive alternative.
The development reflects a growing trend toward patient-centric, minimally invasive therapies—technologies that quietly reshape how we manage everyday pain. Have you considered how such innovations might reduce the burden on oral medication supply chains?
Summary Table:
| Feature | Advantage |
|---|---|
| Market Launch | Expected in ~2 years (regulatory & manufacturing scale-up) |
| Drug Load Capacity | 5-10x higher than current patches/gels for stronger localized effects |
| Adhesion Performance | Stays secure even at 30% drug concentration (by weight) |
| Design | Transparent and discreet for improved patient compliance |
| Therapeutic Use | Targets chronic pain (arthritis, neuralgia) with fewer systemic side effects |
Stay ahead of the curve with Enokon’s cutting-edge transdermal solutions! As a bulk manufacturer of reliable pain relief patches, we specialize in high-drug-load formulations and custom R&D for healthcare distributors and brands. Contact us today to discuss partnerships or early access to innovative pain management technologies.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.

















