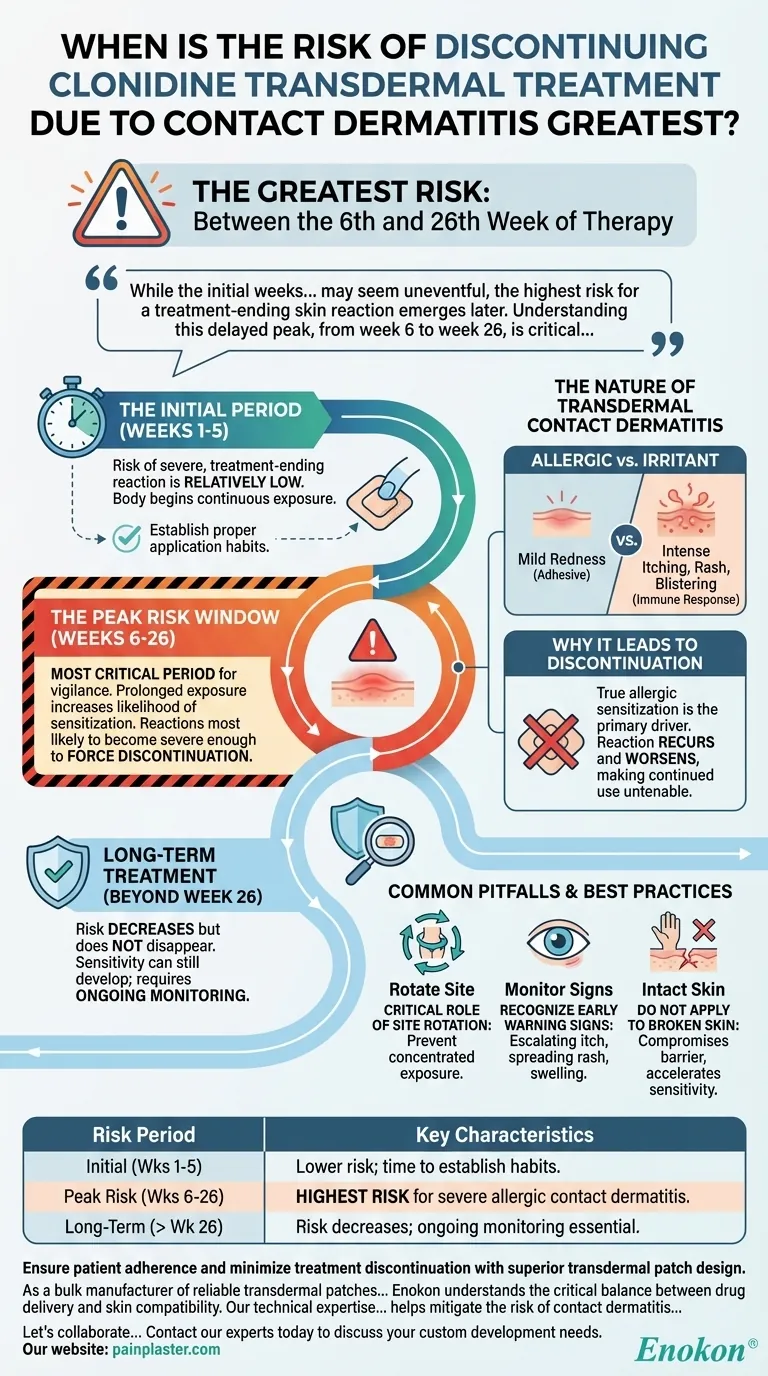
The greatest risk of discontinuing clonidine transdermal treatment due to contact dermatitis occurs between the 6th and 26th week of therapy. While skin sensitivity can arise at any time, this specific period represents the peak window for developing a reaction severe enough to halt treatment.
While the initial weeks of clonidine patch therapy may seem uneventful, the highest risk for a treatment-ending skin reaction emerges later. Understanding this delayed peak, from week 6 to week 26, is critical for proactive patient monitoring and successful long-term management.
Understanding the Timeline of Risk
The development of contact dermatitis from the clonidine patch is not typically an immediate event. The risk evolves over the course of treatment, following a distinct pattern.
The Initial Period (Weeks 1-5)
In the first several weeks of therapy, the risk of a severe, treatment-ending skin reaction is relatively low. The body is just beginning its continuous exposure to the patch and its adhesive components.
The Peak Risk Window (Weeks 6-26)
This is the most critical period for vigilance. The prolonged exposure increases the likelihood of the immune system becoming sensitized to an element of the patch, leading to an allergic contact dermatitis.
It is during this window that reactions are most likely to develop and become severe enough to force the discontinuation of the transdermal system.
Long-Term Treatment (Beyond Week 26)
While the risk is greatest between weeks 6 and 26, it does not disappear. Sensitivity can still develop later in treatment, requiring ongoing monitoring of the skin at the application site.
The Nature of Transdermal Contact Dermatitis
Understanding why this reaction occurs helps in managing the risk. It's a localized response to the components of the patch being in constant contact with the skin.
Allergic vs. Irritant Dermatitis
Contact dermatitis can be either an irritant reaction (mild redness from the adhesive) or a true allergic reaction. An allergic reaction is an immune response that tends to be more severe, with intense itching, rash, and sometimes blistering.
Why It Leads to Discontinuation
A true allergic sensitization is the primary driver for discontinuing treatment. The reaction will recur and often worsen with each new patch application, making continued use untenable for the patient due to significant discomfort and skin damage.
Common Pitfalls and Best Practices
Proactive management can help mitigate the risk of skin reactions and improve the chances of continuing therapy successfully.
The Critical Role of Site Rotation
Failing to rotate the patch application site is a common mistake. Applying the patch to the exact same spot repeatedly concentrates the exposure and significantly increases the risk of local skin irritation and sensitization.
Recognizing Early Warning Signs
Patients and clinicians should monitor for early signs at the patch site. Mild redness is common, but escalating itchiness, spreading rash, or swelling are indicators of a developing problem that needs to be addressed.
Do Not Apply to Broken Skin
Never apply the patch to irritated, broken, or damaged skin. This compromises the skin barrier and can accelerate the development of a sensitivity reaction.
Making the Right Choice for Your Goal
Managing this risk requires vigilance that is timed to the specific phase of treatment.
- If your primary focus is initiating treatment (Weeks 1-5): Establish a strict site rotation schedule and ensure the patient understands how to apply the patch to clean, dry, and intact skin.
- If your primary focus is navigating the peak risk period (Weeks 6-26): Increase vigilance by carefully inspecting the old patch site for any signs of reaction each time a new patch is applied.
- If your primary focus is maintaining long-term therapy (Beyond Week 26): Continue consistent site rotation and monitoring, as the risk of developing a new sensitivity, while lower, still exists.
Proactive monitoring and proper application are key to minimizing the risk of contact dermatitis and maintaining successful long-term clonidine therapy.
Summary Table:
| Risk Period | Key Characteristics |
|---|---|
| Initial (Weeks 1-5) | Lower risk; time to establish proper application habits. |
| Peak Risk (Weeks 6-26) | Highest risk for severe, treatment-ending allergic contact dermatitis. |
| Long-Term (Beyond Week 26) | Risk decreases but ongoing monitoring and site rotation are still essential. |
Ensure patient adherence and minimize treatment discontinuation with superior transdermal patch design.
As a bulk manufacturer of reliable transdermal patches for leading healthcare and pharma brands, Enokon understands the critical balance between drug delivery and skin compatibility. Our technical expertise in custom R&D focuses on developing formulations and adhesives that help mitigate the risk of contact dermatitis, supporting successful long-term therapy for your patients.
Let's collaborate to enhance your transdermal product line. Contact our experts today to discuss your custom development needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints















