To be clear, the methylphenidate transdermal patch is considered habit-forming because the drug it delivers, methylphenidate, is a central nervous system stimulant. It alters brain chemistry by increasing levels of dopamine and norepinephrine. Overuse can cause the brain to adapt, leading to tolerance where larger amounts are needed for the same effect, and creating a cycle of psychological dependence.
The habit-forming potential lies not in the patch delivery system, but in the fundamental way methylphenidate interacts with the brain's reward and attention pathways. Understanding this mechanism is key to using the medication safely and effectively.
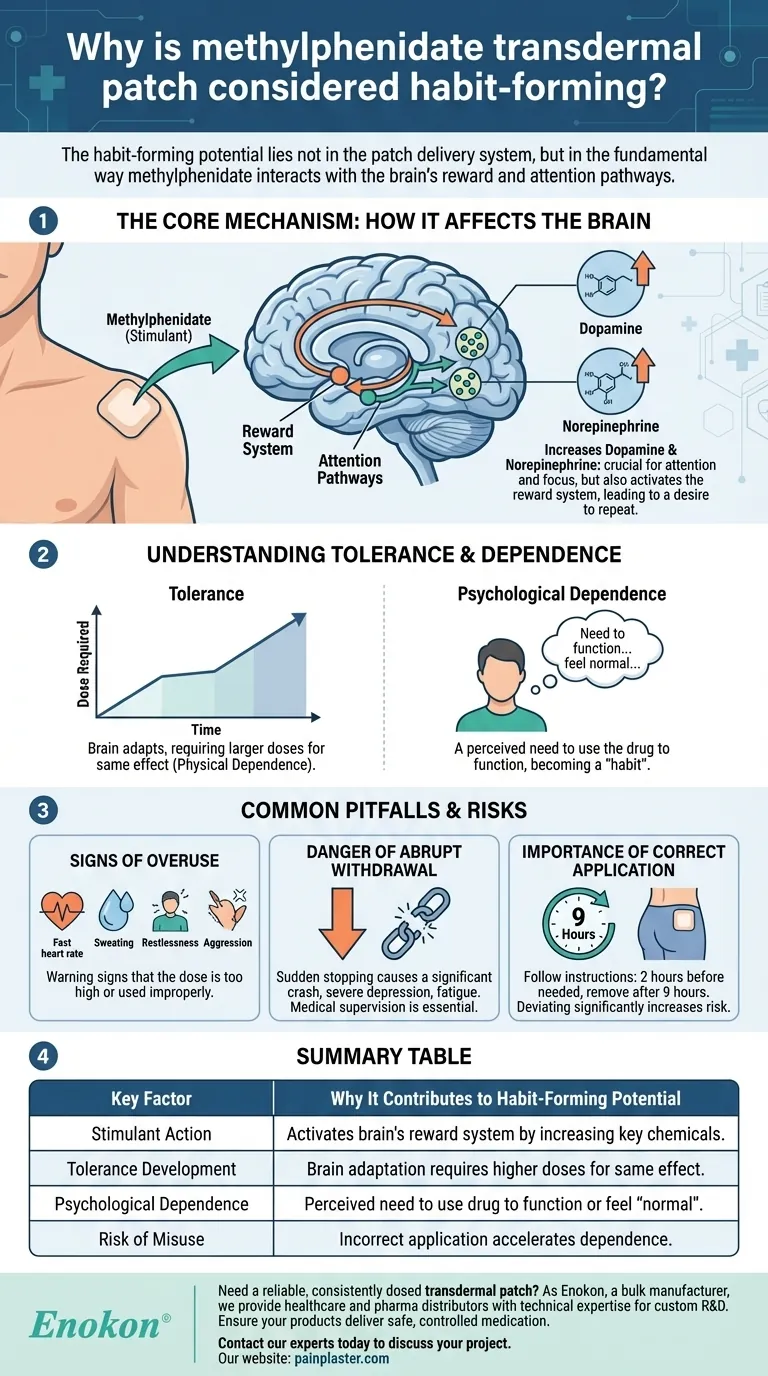
The Core Mechanism: How Methylphenidate Affects the Brain
A Central Nervous System Stimulant
Methylphenidate belongs to a class of medications known as stimulants. These drugs work by increasing the activity of the central nervous system.
Boosting Key Brain Chemicals
The primary action of methylphenidate is to increase the availability of two key neurotransmitters: dopamine and norepinephrine.
These chemicals are crucial for regulating attention, focus, and impulse control, which is why the medication is effective for conditions like ADHD.
Activating the Brain's Reward System
Dopamine is also a central component of the brain's reward system. By increasing dopamine, methylphenidate can create feelings of focus and well-being.
This activation of the reward pathway is the fundamental reason why stimulants carry a risk of being habit-forming. The brain learns to associate the drug with a positive state, leading to a desire to repeat the experience.
Understanding Tolerance and Dependence
What is Tolerance?
With continued use, especially if the dose is higher than prescribed, the brain can begin to adapt to the drug's presence.
This adaptation can lead to tolerance, a state where you need larger or more frequent doses to achieve the original therapeutic effect. This is a critical sign that physical dependence is developing.
Psychological Dependence
Beyond physical tolerance, users can develop a psychological dependence. This is a perceived need to use the drug to function normally, concentrate, or feel "right."
This forms the "habit" itself, where the medication becomes a crutch rather than a tool, often leading to the overuse mentioned in medical warnings.
Common Pitfalls and Risks
Signs of Overuse
Misusing the patch can lead to clear physical and psychological symptoms. These are warning signs that the dose is too high or the drug is being used improperly.
Symptoms can include a fast or irregular heartbeat, sweating, restlessness, agitation, and aggression. In severe cases, it can even lead to thoughts of self-harm.
The Danger of Abrupt Withdrawal
Because the brain adapts to the drug's presence, stopping it suddenly can cause a significant crash.
This withdrawal can manifest as severe depression, fatigue, and other disruptive symptoms. It is why medical supervision is essential when discontinuing the medication.
The Importance of Correct Application
The patch is designed for slow, controlled release. It should be applied to the hip about two hours before the effect is needed and removed after nine hours.
Deviating from these instructions or using more than one patch at a time constitutes misuse and significantly increases the risk of dependence and adverse effects.
Making the Right Choice for Your Health
To manage the risks while benefiting from the treatment, it is critical to adhere strictly to your doctor's guidance.
- If your primary focus is safe and effective treatment: Follow the prescribed dosage and application instructions without fail, including alternating the application site on your hip daily.
- If your primary focus is preventing dependence: Maintain open communication with your doctor about any urges to increase the dose or changes in your mood and behavior.
- If you are considering stopping the medication: Never do it alone. Work with your physician to create a gradual tapering plan to avoid severe withdrawal symptoms.
Understanding how methylphenidate works empowers you to use it as an effective tool while respecting its potential for dependence.
Summary Table:
| Key Factor | Why It Contributes to Habit-Forming Potential |
|---|---|
| Stimulant Action | Increases dopamine & norepinephrine, activating the brain's reward system. |
| Tolerance Development | Brain adapts, requiring larger doses for the same effect, leading to physical dependence. |
| Psychological Dependence | Creates a perceived need to use the drug to function or feel "normal." |
| Risk of Misuse | Incorrect application (e.g., multiple patches) accelerates dependence and adverse effects. |
Need a reliable, consistently dosed transdermal patch? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors with the technical expertise for custom R&D and development. Ensure your products deliver safe, controlled medication. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- Is menthol topical safe during pregnancy and breastfeeding? Key Safety Insights
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained















