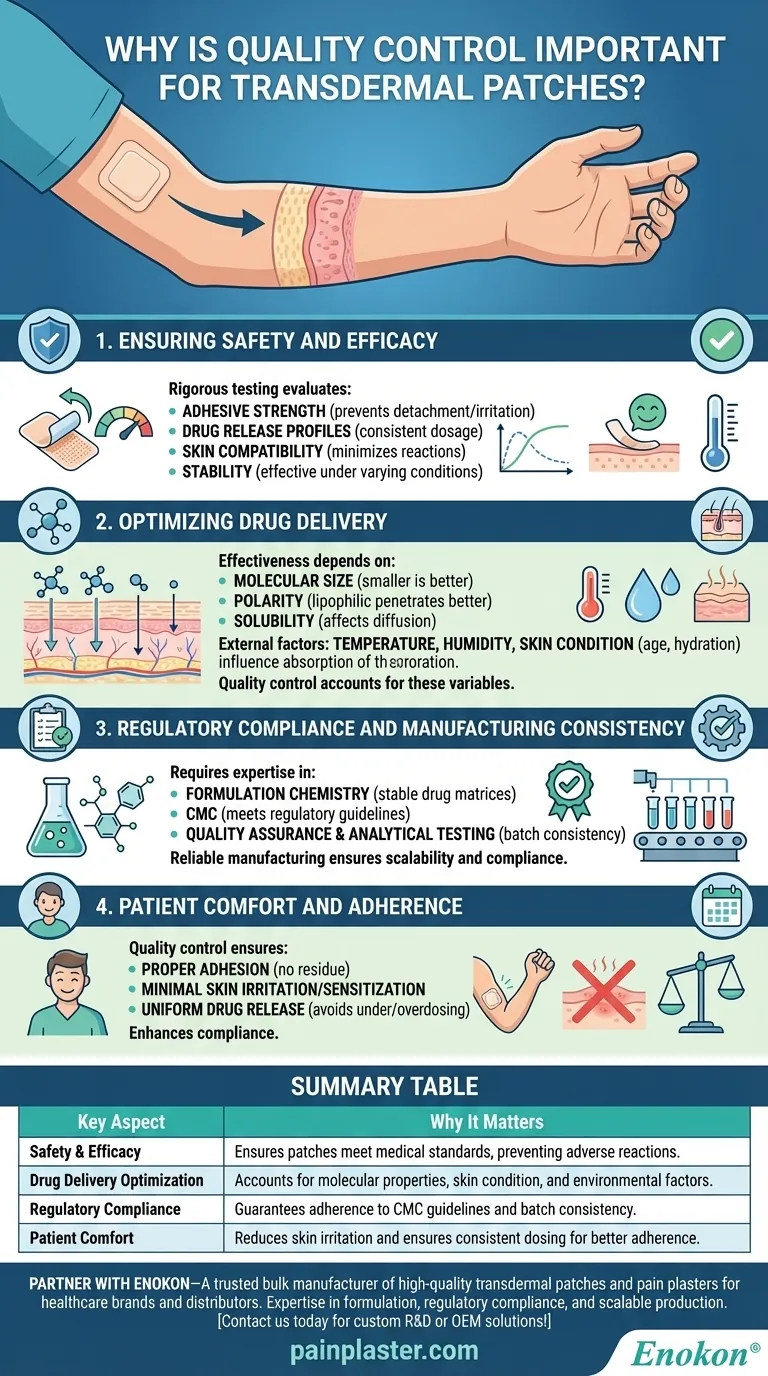
Transdermal patches are a critical drug delivery system that requires stringent quality control to ensure safety, efficacy, and patient comfort. Quality control processes verify adhesive strength, drug release consistency, skin compatibility, and stability, which are essential for reliable therapeutic effects. Factors like molecular properties, skin condition, and environmental influences must also be considered during design and testing. Additionally, expertise in formulation, manufacturing, and regulatory compliance ensures that patches meet industry standards from development to commercial production.

Key Points Explained:
1. Ensuring Safety and Efficacy
- Quality control guarantees that transdermal patches adhere to regulatory and medical standards.
- Rigorous testing evaluates:
- Adhesive strength to prevent premature detachment or skin irritation.
- Drug release profiles to confirm consistent dosage delivery over time.
- Skin compatibility to minimize allergic reactions or irritation.
- Stability to ensure the patch remains effective under varying storage conditions.
2. Optimizing Drug Delivery
- The effectiveness of transdermal patches depends on:
- Molecular size: Smaller molecules absorb more efficiently.
- Polarity: Lipophilic (fat-soluble) drugs penetrate the skin more effectively.
- Solubility: Affects how well the drug dissolves and diffuses through the skin.
- External factors like temperature, humidity, and skin condition (e.g., age, hydration) also influence absorption rates.
- Quality control ensures patches are designed to account for these variables, maximizing therapeutic outcomes.
3. Regulatory Compliance and Manufacturing Consistency
- Developing transdermal patches requires expertise in:
- Formulation chemistry to create stable, effective drug matrices.
- Chemistry, Manufacturing, and Controls (CMC) to meet regulatory guidelines.
- Quality assurance and analytical testing to verify batch consistency.
- A reliable manufacturing partner ensures scalability and compliance from feasibility studies to full-scale production.
4. Patient Comfort and Adherence
- Poorly designed patches can cause discomfort, reducing patient compliance.
- Quality control checks ensure:
- Proper adhesion without excessive residue.
- Minimal skin irritation or sensitization.
- Uniform drug release to avoid under- or overdosing.
By maintaining strict quality control, manufacturers can deliver transdermal patches that are safe, effective, and comfortable for long-term use—key factors in successful treatment outcomes.
Summary Table:
| Key Aspect | Why It Matters |
|---|---|
| Safety & Efficacy | Ensures patches meet medical standards, preventing adverse reactions. |
| Drug Delivery Optimization | Accounts for molecular properties, skin condition, and environmental factors. |
| Regulatory Compliance | Guarantees adherence to CMC guidelines and batch consistency. |
| Patient Comfort | Reduces skin irritation and ensures consistent dosing for better adherence. |
Partner with Enokon—a trusted bulk manufacturer of high-quality transdermal patches and pain plasters for healthcare brands and distributors. Our expertise in formulation, regulatory compliance, and scalable production ensures your patches meet the highest standards. Contact us today for custom R&D or OEM solutions!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
















