To put it simply, you must remove a scopolamine patch before an MRI because it contains a thin metallic layer. The powerful energy used during an MRI can heat this metal to a very high temperature, creating a significant risk of a serious skin burn at the patch site.
The core issue is not the medication itself, but the physical construction of the patch. The radiofrequency energy from the MRI scanner can induce an electrical current in the patch's hidden metal layer, turning it into a small heating element directly on your skin.
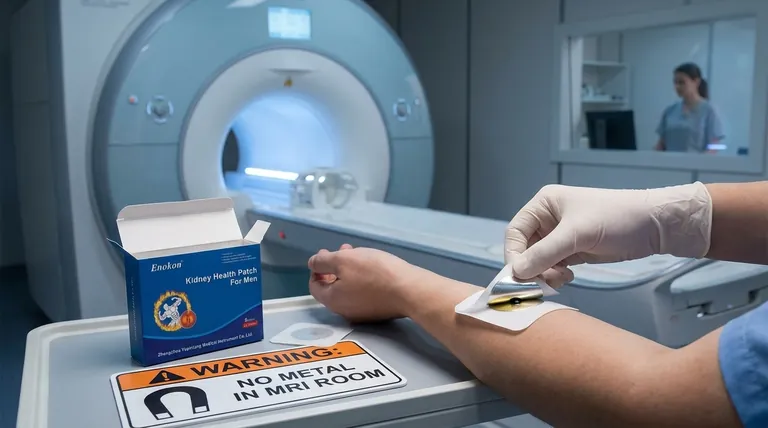
How an MRI Creates the Burn Risk
To understand the danger, you need to understand two key components of an MRI scan and how they interact with metal.
The Radiofrequency (RF) Pulses
While most people know MRIs use powerful magnets, the heating effect is primarily caused by the radiofrequency (RF) pulses. These are bursts of energy used to create the detailed images of your body.
The Problem with Conductive Metals
When this RF energy passes through a conductive material, like the metal in a patch, it can induce powerful electrical currents. These are often called eddy currents.
This process is similar to how an induction cooktop works. The cooktop generates an electromagnetic field that induces currents in the metal pot, causing the pot itself to heat up and cook the food.
Deconstructing the Scopolamine Patch
The risk becomes clear when you look at how many transdermal patches are made.
The Hidden Metallic Layer
Many patches, including common brands of scopolamine, incorporate a very thin layer of metal, often aluminum. This layer is not always obvious to the naked eye.
Why Is Metal in the Patch?
This metallic layer serves a functional purpose. It often acts as a backing to prevent the drug from evaporating or leaking out the back, ensuring it is delivered through the skin as intended.
Understanding the Potential Injury
Failing to remove a metallic patch before an MRI is not a minor oversight; it can lead to genuine harm.
The Mechanism of the Burn
The eddy currents generated in the patch's metal layer create intense, localized heat. Because the patch is held tightly against your skin, this heat has nowhere to go and is transferred directly to your tissue.
Severity of the Injury
This is not just a mild irritation. The Food and Drug Administration (FDA) has received reports of patients suffering first, second, and even third-degree burns from wearing metallic patches during an MRI.
This Risk Goes Beyond Scopolamine
It is critical to understand that this risk is not unique to scopolamine. Any transdermal patch containing a metallic component poses the same danger. This includes certain patches for nicotine, fentanyl, hormones, and other medications.
How to Prepare for Your MRI
Clear communication with your healthcare team is the most effective way to ensure your safety.
- If you are preparing for an MRI: You must inform the radiologist and MRI technologist about all medications you use, including any creams, ointments, or transdermal patches.
- If you are directed to remove a patch: Carefully peel it off before the scan, clean the area with soap and water, and be sure to apply a new patch to a different clean, dry area after your appointment is over.
- If you are ever unsure: Always ask your doctor or pharmacist if your specific brand of patch contains metal before undergoing an MRI.
Ultimately, being an active and informed participant in your own care is the best way to prevent this avoidable injury.
Summary Table:
| Risk Factor | Consequence | Prevention |
|---|---|---|
| Hidden Metallic Layer | Intense localized heating | Remove patch before scan |
| MRI Radiofrequency Pulses | Induces electrical currents (eddy currents) | Inform technologist of all patches |
| Direct Skin Contact | Potential for 1st, 2nd, or 3rd-degree burns | Clean area with soap and water after removal |
Ensure Patient Safety with Non-Metallic Patch Solutions
As a healthcare distributor or brand, patient safety is paramount. The risk of MRI burns from metallic patches is a serious concern that can be mitigated with the right patch design.
Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise allows for custom R&D and development of patches that prioritize patient safety without compromising on drug delivery efficacy.
Let's collaborate to develop safer, innovative transdermal solutions for your patients.
Contact our experts today to discuss your needs
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What should be done in case of a testosterone patch overdose? A Step-by-Step Emergency Guide
- How often should testosterone patches be applied? Daily Dosage & Best Practices
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment
- What precautions should be taken when applying testosterone patches? Maximize Safety and Effectiveness
- What should be done before undergoing an MRI while using testosterone patches? Remove it to prevent serious burns.
















