In short, the transdermal form of oxybutynin was developed for a single, critical reason: to reduce the significant side effect of dry mouth associated with the oral version of the medication. By delivering the drug through the skin, it changes how the body processes it, targeting the direct cause of this common and often intolerable side effect.
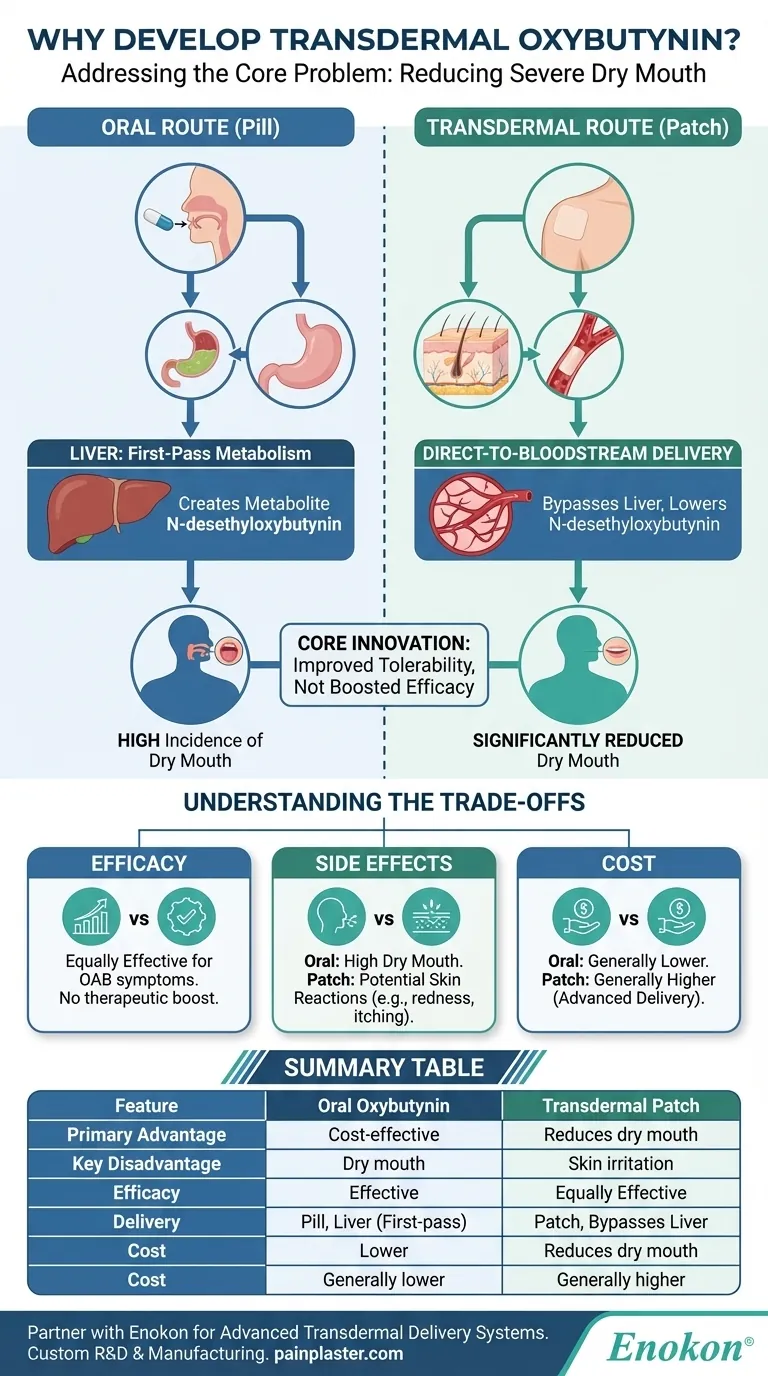
The core innovation of the oxybutynin patch is not in boosting its effectiveness but in improving its tolerability. It was engineered to bypass the liver, thereby minimizing the creation of a metabolite responsible for causing severe dry mouth.
The Core Problem with Oral Oxybutynin
To understand the patch, we must first look at the limitations of the pill. While effective for treating an overactive bladder, the oral route creates an unavoidable biochemical problem.
How Oral Medication Works
When you take oxybutynin orally, it is absorbed through your digestive system. It belongs to a class of drugs called antimuscarinics, which work by relaxing the bladder's smooth muscle to reduce the urgent and frequent need to urinate.
The Role of First-Pass Metabolism
After absorption, the drug travels directly to the liver. Here, it undergoes a process called first-pass metabolism, where a significant portion of the drug is chemically altered before it ever reaches the rest of the body.
The Metabolite Behind the Discomfort
This liver metabolism creates a new compound called N-desethyloxybutynin. Researchers discovered that this specific active metabolite is the primary culprit behind the intense dry mouth that many patients experience with oral oxybutynin.
The Transdermal Solution: Bypassing the Liver
The transdermal patch was designed specifically to circumvent this metabolic pathway and its problematic side effect.
Direct-to-Bloodstream Delivery
A transdermal patch delivers oxybutynin directly through the skin into the bloodstream. This allows for a steady, continuous release of the medication over several days.
Reducing the Problematic Metabolite
By entering the bloodstream directly, the drug avoids the first-pass effect in the liver. This dramatically reduces the amount of N-desethyloxybutynin produced compared to the oral forms.
The Impact on Side Effects
With significantly lower levels of the problematic metabolite circulating in the body, patients experience a much lower incidence of dry mouth, making the treatment far more tolerable for long-term use.
Understanding the Trade-offs
While the patch successfully reduces one major side effect, this benefit comes with its own set of considerations. It is a re-engineered solution, not a superior one in every aspect.
Efficacy: Is It More Effective?
No. The transdermal patch is no more effective at controlling overactive bladder symptoms than the short- or long-acting oral forms. The therapeutic benefit for the bladder remains the same.
A New Side Effect: Skin Reactions
The patch introduces a new potential side effect: skin irritation. Application site reactions, like redness or itching, are common and will cause approximately 10% of patients to discontinue using it.
The Cost Factor
The transdermal patch is a more advanced delivery system and, as a result, generally costs more than its generic oral counterparts. This can be a significant factor in long-term treatment decisions.
Making the Right Choice for Your Condition
Choosing between oral and transdermal oxybutynin requires weighing the trade-off between side effect profiles and cost.
- If your primary focus is symptom relief at the lowest possible cost: The oral form is often the first-line treatment, provided you can tolerate the potential for dry mouth.
- If your primary focus is avoiding severe dry mouth: The transdermal patch offers a direct and effective solution, assuming you do not experience significant skin irritation and the higher cost is manageable.
Ultimately, the development of the oxybutynin patch provides a valuable alternative that prioritizes patient comfort and adherence to treatment.
Summary Table:
| Feature | Oral Oxybutynin | Transdermal Oxybutynin Patch |
|---|---|---|
| Primary Advantage | Cost-effective, first-line treatment | Significantly reduces dry mouth side effect |
| Key Disadvantage | High incidence of dry mouth | Potential for skin irritation at application site |
| Efficacy for OAB | Effective | Equally Effective |
| Delivery Method | Pill, passes through liver (first-pass metabolism) | Patch, bypasses liver, direct to bloodstream |
| Cost | Generally lower (generic available) | Generally higher |
Struggling with side effects from your current medication formulation?
At Enokon, we specialize in developing advanced transdermal delivery systems that can improve patient tolerance and adherence. As a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharma distributors and brands to create custom solutions.
Benefit from our technical expertise for your custom R&D and product development needs. Let's discuss how a tailored transdermal patch can enhance your product line and provide a better experience for patients.
Contact our experts today to explore a partnership!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief














