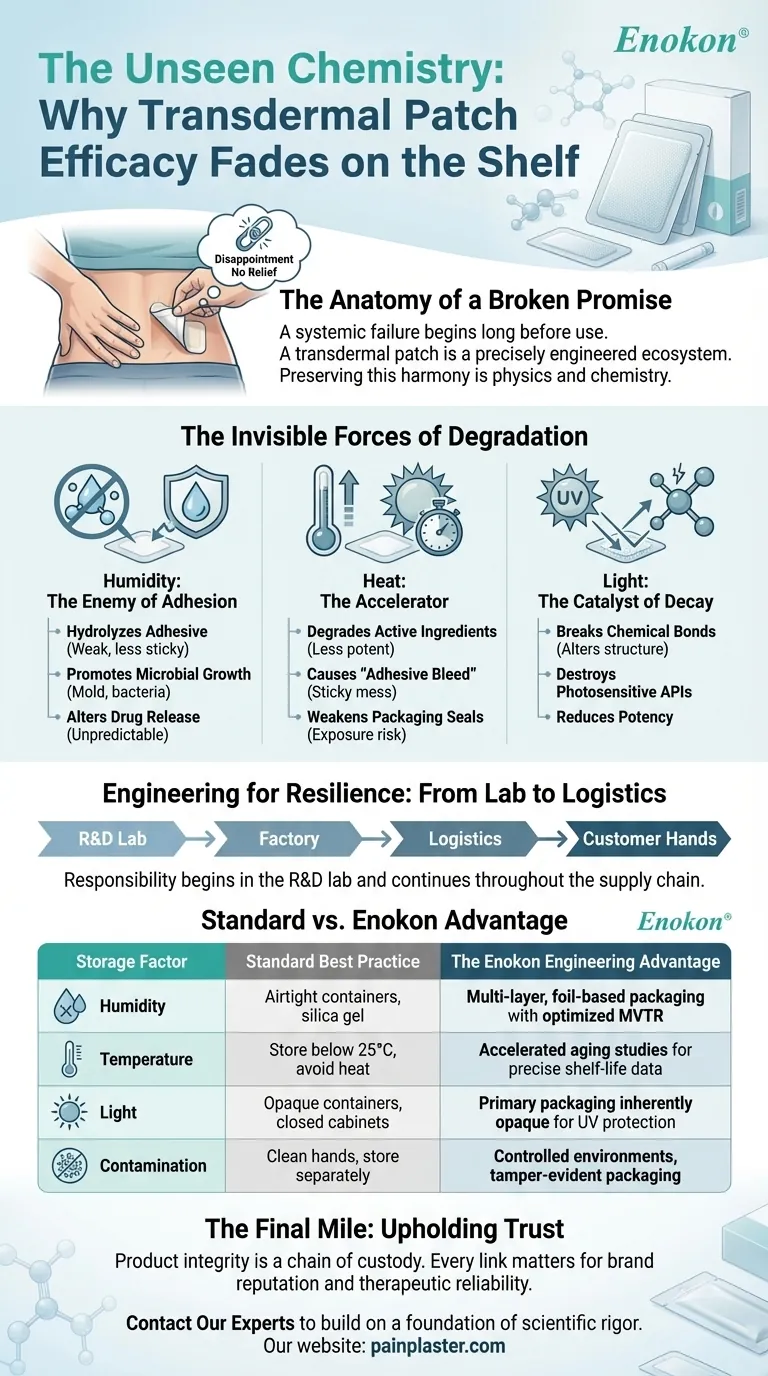
The Anatomy of a Broken Promise
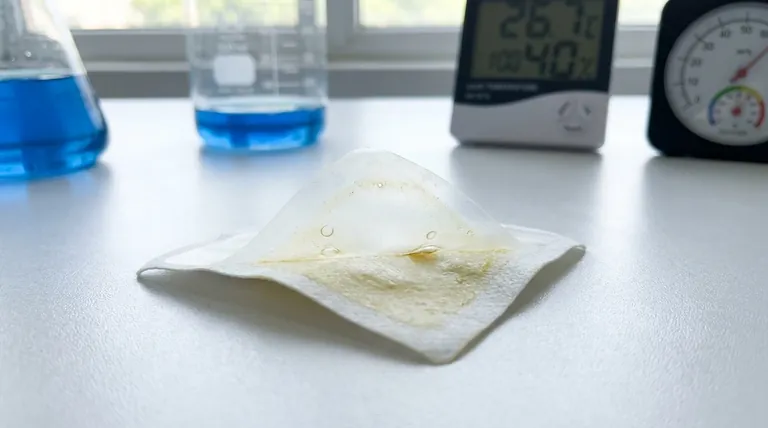
Imagine a customer, seeking relief from persistent back pain. They open a pain plaster they’ve trusted, but today something is wrong. The patch is stiff, the adhesive feels weak, and it peels off within an hour. The expected relief never arrives.
This small, personal moment of disappointment isn't an isolated incident. It's the end result of a systemic failure that began months, or even years, earlier. The promise of the product was broken long before the package was opened, a victim of unseen forces.
A transdermal patch is not merely a medicated sticker. It is a precisely engineered ecosystem. The backing layer, the adhesive matrix, the active pharmaceutical ingredients (APIs), and the release liner are all designed to work in perfect harmony. Preserving that harmony is a matter of physics and chemistry.
The Invisible Forces of Degradation
We tend to think of sealed products as static, existing in a state of suspended animation until we use them. The reality is that they are in a constant, slow-motion battle with their environment. For a transdermal patch, the primary adversaries are humidity, heat, and light.
The Enemy of Adhesion: Humidity
Moisture is the silent saboteur of a patch’s physical integrity. The adhesives used in medical patches are complex polymers designed for biocompatibility and specific tackiness. When water molecules from the air penetrate the packaging, they can:
- Hydrolyze the Adhesive: Breaking down the polymer chains, causing the adhesive to become weak and lose its stickiness.
- Promote Microbial Growth: Creating a breeding ground for mold and bacteria, compromising safety and efficacy.
- Alter Drug Release: Swelling the patch's matrix, which can unpredictably change the rate at which the active ingredient is delivered to the skin.
We feel humidity, but we don't see its molecular effect until the moment of failure—when the patch refuses to stick.
The Accelerator: Heat
Heat is an accelerator. It speeds up every chemical reaction, including the ones that degrade the product. Storing plasters near a radiator, in a sun-baked warehouse, or inside a hot delivery truck can be catastrophic.
High temperatures can:
- Degrade Active Ingredients: Breaking down the complex herbal or pharmaceutical molecules, rendering the patch less potent.
- Cause "Adhesive Bleed": Making the adhesive melt and flow, creating a sticky mess and compromising the patch's structure.
- Weaken Packaging Seals: Increasing the risk of exposure to other environmental factors.
A simple law of thermodynamics has massive consequences for product reliability and brand reputation.
The Catalyst of Decay: Light
Sunlight, particularly its UV component, is a powerful catalyst for chemical degradation. Many APIs are photosensitive. Exposure to light can break chemical bonds, altering the molecular structure of the very ingredients meant to provide relief. This is why preserving the potency of the formula requires shielding it from light at every stage.
Engineering for Resilience: From Lab to Logistics
The responsibility for product stability doesn't start with the end-user. It begins in the R&D lab and is upheld throughout the entire supply chain. For healthcare distributors and brands, the choice of a manufacturing partner is a choice about reliability.
At Enokon, we don't just formulate effective patches; we engineer them for resilience. We anticipate these environmental challenges because our expertise lies in understanding the complex interplay between the formulation, the materials, and the packaging.
This holistic approach is critical for maintaining product integrity from our factory to your customer's hands.
| Storage Factor | Standard Best Practice | The Enokon Engineering Advantage |
|---|---|---|
| Humidity | Use airtight containers, add silica gel | We select multi-layer, foil-based packaging with optimized Moisture Vapor Transmission Rates (MVTR) to create a stable micro-climate for each patch. |
| Temperature | Store below 25°C (77°F), avoid heat sources | Our R&D includes accelerated aging studies to define precise, reliable shelf-life data for different climate zones, informing your logistics. |
| Light | Keep in opaque containers or closed cabinets | Our primary packaging is inherently opaque, providing a robust barrier against UV radiation to protect photosensitive active ingredients. |
| Contamination | Handle with clean hands, store separately | We manufacture in controlled environments and use packaging designed to be tamper-evident and impervious to external contaminants. |
The Final Mile: Upholding Trust
The integrity of your brand rests on the consistent performance of your product. When a distributor or pharmaceutical company partners with a manufacturer, they are outsourcing a critical piece of that trust.
The meticulous attention to material science, stability testing, and packaging engineering isn't just a technical requirement. It is the foundation of a reliable therapeutic experience and the safeguard of your reputation. Product efficacy is a chain of custody, and every link matters.
For brands and distributors who understand that product integrity is non-negotiable, ensuring every link in that chain is strong is paramount. To build your product line on a foundation of scientific rigor and manufacturing excellence, Contact Our Experts.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
Related Articles
- More Than a Feeling: The Precise Engineering of Cold vs. Hot Pain Relief
- Beyond the Molecule: The Behavioral Science of the Simple Pain Patch
- Beyond the 'Ouch': The Delicate Engineering of Pediatric Pain Patches
- Beyond the Pill: The Engineering and Psychology of Transdermal Pain Relief in Dentistry
- The Precision Imperative: Why Topical NSAIDs Outsmart Oral Painkillers