Crucially, there are no documented severe, serious, moderate, or mild drug interactions listed for capsaicin transdermal patches. While this suggests a low risk of medication interference, it does not mean the treatment is without risk. The primary safety concerns revolve around significant local side effects and the potential for increased blood pressure.
The absence of drug interactions shifts the safety focus entirely to proper application and monitoring. The most important considerations are managing intense skin reactions at the application site and observing for potential cardiovascular effects, particularly changes in blood pressure.
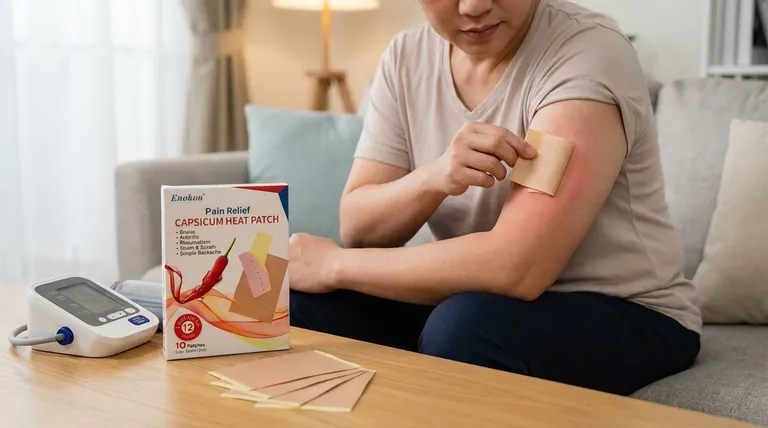
The Core Safety Profile of Transdermal Capsaicin
Understanding how capsaicin works explains its unique safety profile. Its action is highly localized, which minimizes the potential for systemic drug interactions.
How Capsaicin Works Locally
The capsaicin in the patch is absorbed into the skin where it targets local nerve endings. It works by overwhelming these nerves and depleting a chemical called substance P, which is responsible for sending pain signals to the brain. Because its primary effect is confined to the application area, very little of the drug enters the bloodstream.
A Lack of Systemic Interference
This localized mechanism is why no significant drug-on-drug interactions have been identified. The treatment does not typically reach high enough concentrations in the blood to interfere with other medications processed by the liver or kidneys.
The Real Risks: Side Effects and Precautions
While drug interactions are not a primary concern, you must be aware of the common and potential side effects to use this treatment safely.
Common Application Site Reactions
The most frequent side effects occur directly where the patch is applied. These are an expected consequence of how the drug works and can include transient increased pain, redness, itching, swelling, dryness, and the formation of small bumps (papules).
Potential Systemic Effects
Although rare, some of the medication can be absorbed systemically, leading to other effects. The most significant is a temporary increase in blood pressure (hypertension), which typically occurs shortly after application. Other reported effects include nausea, vomiting, headache, and coughing.
Severe but Rare Reactions
In a small number of cases, more severe reactions can occur. These may include skin burns, blisters, or even permanent scarring at the application site. Neurological symptoms like dizziness or reduced sensation have also been noted.
Understanding Key Precautions
Proper handling and awareness of specific warnings are critical for mitigating the risks associated with capsaicin transdermal therapy.
Monitor Blood Pressure
Due to the risk of hypertension, blood pressure should be monitored, especially within the first hour after the patch is applied. This is particularly important for individuals with a history of uncontrolled hypertension or previous cardiovascular events like a stroke.
Avoid Sensitive Areas and Heat
The patch should never be applied to the face, scalp, or broken skin. Additionally, exposing the application area to external heat sources like heating pads or direct sunlight can increase absorption and intensify side effects.
Ensure Careful Application and Removal
When removing the patch, do so gently and slowly. Ripping it off quickly can cause small particles of dried capsaicin to become airborne (aerosolization), which can lead to coughing and irritation of the eyes, nose, and throat.
Prevent Accidental Exposure
The active ingredient is a powerful irritant. It is essential to keep the patch away from children and pets. Avoid touching the patch and then touching your eyes, mouth, or other sensitive areas.
How to Use Capsaicin Transdermal Safely
Your approach should be guided by your specific health profile and treatment goals.
- If your primary focus is managing pain: Be prepared for significant but temporary local skin irritation, as this is the most common side effect you will encounter.
- If you have pre-existing hypertension or heart conditions: Your key takeaway is that you must discuss this treatment with your doctor and ensure your blood pressure is monitored during use.
- If you are concerned about overall safety: The main priority is not drug interactions but rather careful handling of the patch and knowing when to seek medical help for severe skin reactions or systemic symptoms.
Ultimately, using capsaicin transdermal safely means understanding that the real risks are local, not interactive.
Summary Table:
| Safety Aspect | Key Consideration |
|---|---|
| Drug Interactions | No documented severe, moderate, or mild interactions. |
| Primary Risk | Local skin reactions (redness, pain, itching) at the application site. |
| Key Systemic Concern | Potential for temporary increase in blood pressure. |
| Critical Precaution | Avoid application to broken skin, face, and exposure to external heat. |
Need a reliable, high-quality transdermal pain patch? Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Benefit from our technical expertise for custom R&D and development to create a safe and effective product for your market. Contact our experts today to discuss your requirements.
Visual Guide

Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide















