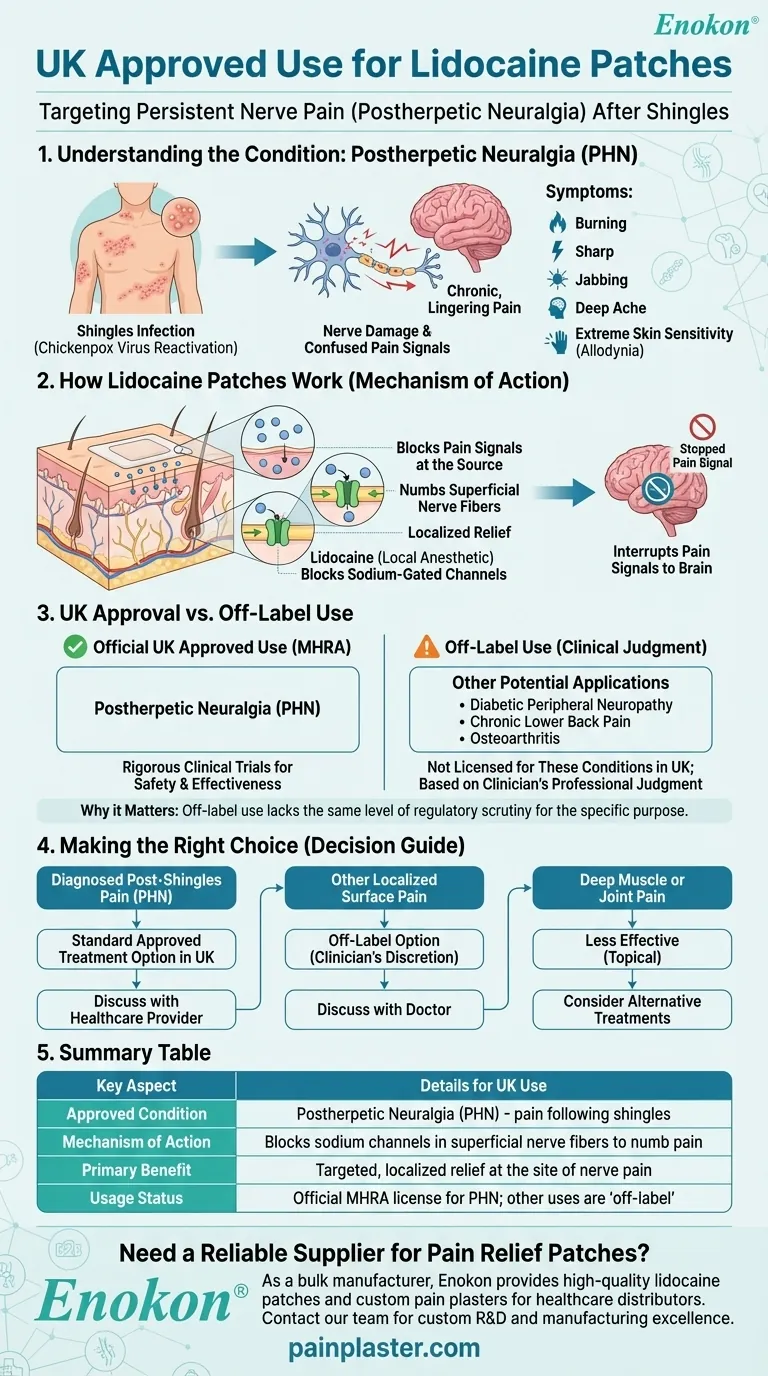
In the United Kingdom, lidocaine patches are officially approved to treat the persistent nerve pain that some people experience after a shingles infection. This specific condition is known medically as postherpetic neuralgia.
The core purpose of a lidocaine patch is to provide targeted, localized pain relief by numbing the superficial nerve fibers in the skin. While its official UK approval is narrow, this mechanism is why it's sometimes considered for other types of localized nerve pain.

Understanding Postherpetic Neuralgia (PHN)
The Lingering Pain from Shingles
Postherpetic neuralgia, or PHN, is a common complication of shingles. Shingles itself is caused by the reactivation of the chickenpox virus.
Even after the shingles rash and blisters have healed, the virus can damage nerve fibers. This damage causes the nerves to send confused and exaggerated pain signals to the brain, resulting in chronic pain.
The Nature of Nerve Pain
The pain from PHN is often described as burning, sharp, jabbing, or a deep ache. The affected skin can also become extremely sensitive to touch, a condition known as allodynia.
How Lidocaine Patches Target This Pain
A Localized Anesthetic Effect
Lidocaine is a local anesthetic. When applied as a patch, it is absorbed through the skin directly at the site of the pain.
Blocking Pain Signals at the Source
The medication works by blocking specific pathways called sodium-gated channels within the pain-sensing nerve fibers just under the skin.
By blocking these channels, the patch interrupts the pain signals being sent from the damaged nerves to the brain, providing localized relief without affecting the entire body.
Common Pitfalls: Approved vs. "Off-Label" Use
The Official UK Approval
In the United Kingdom, the formal license for prescription lidocaine patches is strictly for postherpetic neuralgia (PHN). This means it has undergone rigorous clinical trials specifically for this condition to prove its safety and effectiveness.
Other Potential Applications
Research and clinical practice, particularly in other regions like the United States, have explored using lidocaine patches for other pain conditions. These may include diabetic peripheral neuropathy, chronic lower back pain, and osteoarthritis.
Why the Distinction Matters
When a medication is used for a condition it isn't officially licensed for, this is known as "off-label" use. While legal and sometimes appropriate, it means the treatment has not passed the same level of regulatory scrutiny for that specific purpose. Any such use would be based on a clinician's professional judgment.
Making the Right Choice for Your Goal
To determine if this treatment is relevant for you, consider the nature of your pain.
- If your primary focus is treating diagnosed post-shingles pain: A lidocaine patch is a standard, approved treatment option in the UK to discuss with your healthcare provider.
- If your primary focus is another type of localized surface pain: A doctor might consider a lidocaine patch an "off-label" option, but it falls outside its official UK approval.
- If your primary focus is deep muscle or joint pain: A topical patch may be less effective, as its mechanism is specifically designed to numb the nerve fibers just beneath the skin.
Ultimately, understanding the precise, approved application of lidocaine patches empowers you to have a more informed discussion with your doctor about your specific pain management needs.
Summary Table:
| Key Aspect | Details for UK Use |
|---|---|
| Approved Condition | Postherpetic Neuralgia (PHN) - pain following shingles |
| Mechanism of Action | Blocks sodium channels in superficial nerve fibers to numb pain |
| Primary Benefit | Targeted, localized relief at the site of nerve pain |
| Usage Status | Official MHRA license for PHN; other uses are 'off-label' |
Need a reliable supplier for pain relief patches?
As a bulk manufacturer of transdermal patches, Enokon provides healthcare and pharmaceutical distributors with high-quality, consistent lidocaine patches and custom pain plasters. Our technical expertise supports custom R&D to meet your specific product development needs.
Contact our team today to discuss your requirements and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- What allergic reactions can lidocaine patches cause? Risks & Prevention Tips
- What outcome measures were used to assess the effectiveness of the lidocaine patch 5%? Key Metrics for Pain Relief Evaluation
- What is the role of a rotary evaporator in Lidocaine nano-liposomes? Key to High-Performance Thin-Film Preparation
- What precautions should be taken when disposing of lidocaine patches? Protect Your Household from Accidental Poisoning
- What are the key dosing considerations for lidocaine transdermal patches? Ensure Safe & Effective Pain Relief
- What are the steps for applying a lidocaine plaster? A Complete Guide to Safe and Effective Pain Relief
- How do lidocaine patches differ from Icy Hot and Biofreeze patches in their mechanism of action?
- What was the treatment regimen for the lidocaine patch 5% in the study? Effective Pain Management Protocol
















