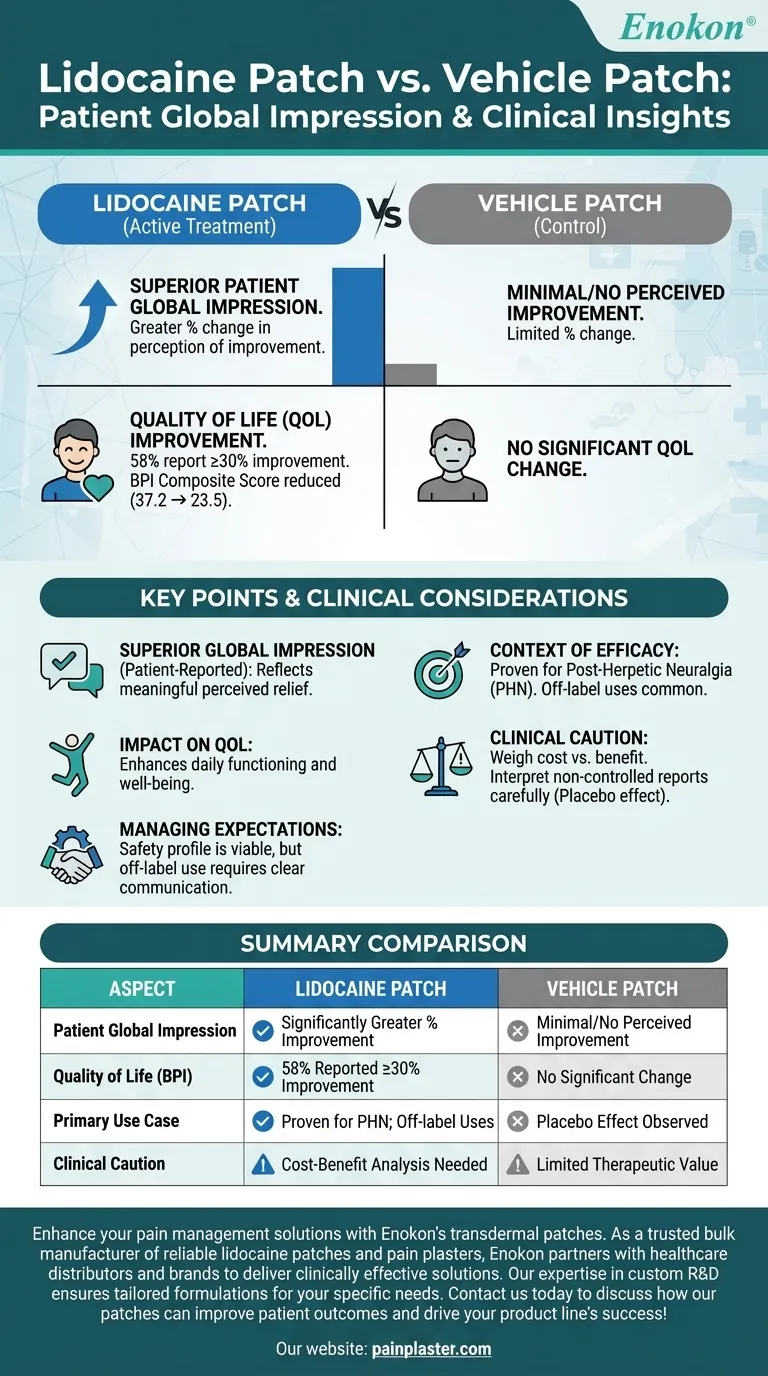
The lidocaine patch demonstrated superior performance compared to the vehicle patch in terms of patient global impression of improvement. Specifically, it showed a greater percentage change in patients' perception of their condition, indicating enhanced effectiveness. This aligns with broader observations about the lidocaine patch's role as a topical analgesic, though its efficacy beyond certain conditions like post-herpetic neuralgia remains debated. The improvement in quality of life scores further supports its potential benefits, though clinicians should remain cautious about interpreting non-controlled reports due to possible placebo effects.

Key Points Explained:
-
Superior Patient Global Impression with Lidocaine Patch
- The lidocaine patch produced a significantly greater percentage change in patients' global impression of improvement compared to the vehicle patch. This suggests that patients perceived greater relief or enhancement in their condition when using the active treatment.
- This metric is crucial as it reflects patient-reported outcomes, which are often more meaningful in clinical practice than purely objective measures.
-
Context of Lidocaine Patch Efficacy
- While the lidocaine patch for pregnancy is widely used off-label for various pain conditions, its proven efficacy is primarily documented for post-herpetic neuralgia (PHN).
- The observed benefits in global impression may partly stem from placebo effects, emphasizing the need for controlled studies to validate its use in other pain syndromes.
-
Impact on Quality of Life (QOL)
- Supporting the global impression data, the BPI composite score showed a notable reduction in QOL impairment (from 37.2 to 23.5), with 58% of patients reporting ≥30% improvement.
- This underscores the patch's potential to enhance daily functioning and overall well-being, even if its mechanism isn't fully understood for all applications.
-
Clinical Considerations and Caution
- Despite positive patient feedback, clinicians should weigh the cost versus benefit, especially given the patch's expense and variable evidence base.
- Non-controlled reports of efficacy (like those from open-label studies) require careful interpretation to avoid overestimating the patch's effectiveness.
-
Balancing Patient Expectations and Evidence
- The lidocaine patch's safety profile makes it a viable option for many, but managing patient expectations is key—especially when used off-label.
- Future research should focus on randomized controlled trials to clarify its role beyond PHN and differentiate true therapeutic effects from placebo responses.
By integrating these insights, healthcare providers can make more informed decisions about when and how to recommend the lidocaine patch, ensuring alignment with both patient needs and evidence-based practice.
Summary Table:
| Aspect | Lidocaine Patch | Vehicle Patch |
|---|---|---|
| Patient Global Impression | Significantly greater % improvement | Minimal/no perceived improvement |
| Quality of Life (BPI) | 58% reported ≥30% improvement | No significant change |
| Primary Use Case | Proven efficacy for PHN; off-label uses | Placebo effect observed |
| Clinical Caution | Cost-benefit analysis needed | Limited therapeutic value |
Enhance your pain management solutions with Enokon’s transdermal patches
As a trusted bulk manufacturer of reliable lidocaine patches and pain plasters, Enokon partners with healthcare distributors and brands to deliver clinically effective solutions. Our expertise in custom R&D ensures tailored formulations for your specific needs.
Contact us today to discuss how our patches can improve patient outcomes and drive your product line’s success!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Prostate Pain Kidney Health Care Patch for Men
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
















