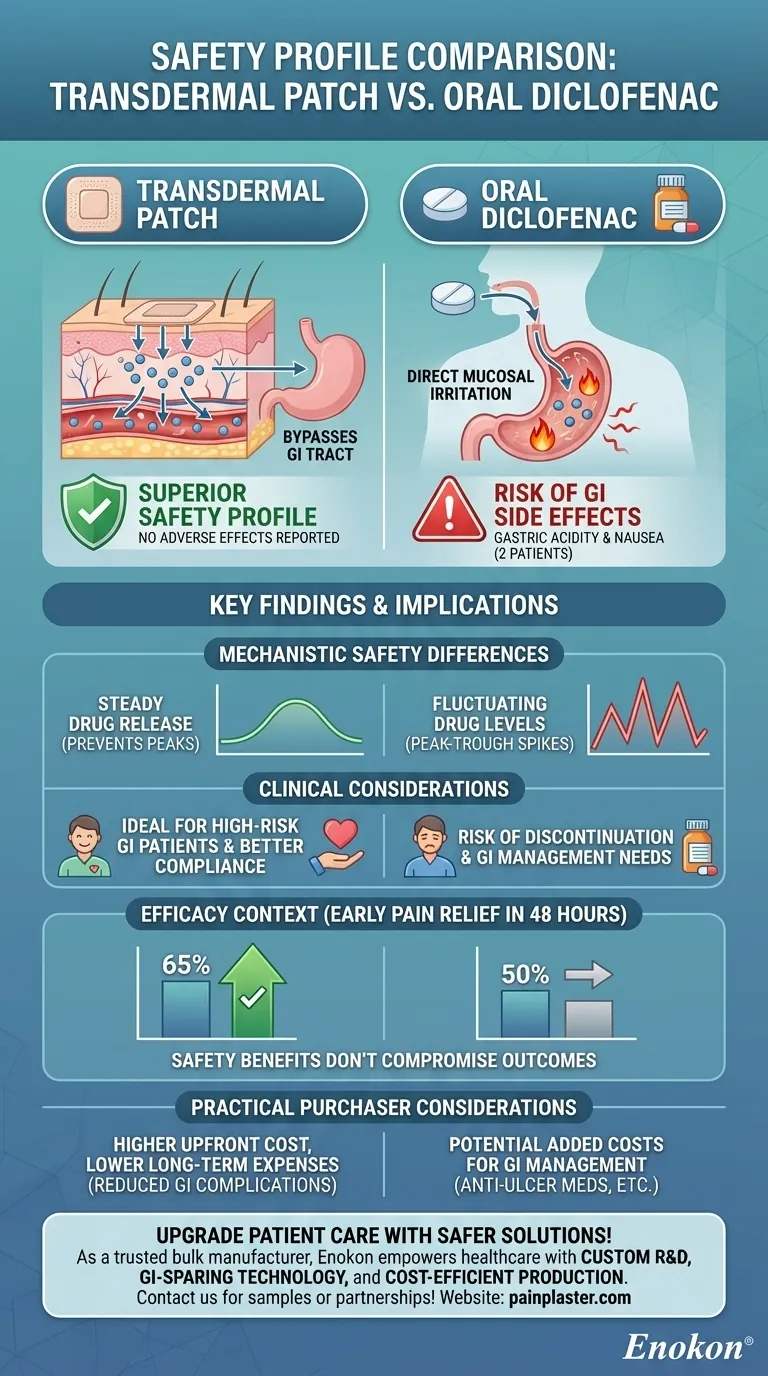
The study comparing transdermal diclofenac patches and oral diclofenac tablets found that the transdermal patch demonstrated a superior safety profile. While no adverse effects were reported for the patch group, two patients on oral therapy experienced gastric acidity and nausea. Both methods provided significant pain relief, but the transdermal delivery system avoided common gastrointestinal side effects associated with oral NSAIDs while maintaining efficacy.
Key Points Explained:
-
Adverse Effects Comparison
- Transdermal Patch: No local or systemic adverse effects were reported in any patient using the patch. This aligns with the inherent advantages of transdermal delivery, such as bypassing the gastrointestinal tract and avoiding first-pass metabolism.
- Oral Diclofenac: Two patients experienced gastric acidity and nausea, likely due to direct mucosal irritation and systemic exposure typical of oral NSAIDs.
-
Mechanistic Reasons for Safety Differences
- GI Tract Avoidance: The patch delivers diclofenac through the skin, eliminating direct contact with the stomach lining—a primary cause of oral NSAID-induced gastritis.
- Steady Drug Levels: Transdermal systems provide consistent drug release, preventing peak-trough fluctuations that can trigger side effects (e.g., rapid spikes in plasma concentrations with oral dosing).
-
Clinical Implications for Patient Care
- High-Risk Patients: The patch may be preferable for patients with a history of GI ulcers or sensitivity to oral NSAIDs.
- Compliance: No adverse effects correlate with better adherence, as patients are less likely to discontinue treatment due to discomfort.
-
Efficacy Context
- Both methods achieved statistically significant pain reduction, with the patch showing marginally higher early pain relief (65% vs. 50% in the first 48 hours). This suggests safety benefits don’t compromise therapeutic outcomes.
-
Practical Considerations for Purchasers
- Cost-Benefit: While patches may have higher upfront costs, reduced adverse events could lower downstream expenses (e.g., antiulcer medications or hospitalizations for GI complications).
- Storage/Handling: Patches require stable storage conditions but eliminate risks like accidental ingestion or dosing errors common with oral tablets.
The findings highlight how delivery systems can redefine drug safety without sacrificing efficacy—an insight valuable for formulary decisions in clinics prioritizing patient comfort. Would your facility benefit from integrating transdermal options for high-risk populations?
Summary Table:
| Aspect | Transdermal Patch | Oral Diclofenac |
|---|---|---|
| Adverse Effects | None reported | Gastric acidity, nausea (2 patients) |
| Delivery Mechanism | Bypasses GI tract; steady drug release | Direct mucosal irritation |
| Patient Preference | Ideal for high-risk GI patients | Risk of discontinuation due to side effects |
| Efficacy | 65% pain relief in 48 hours | 50% pain relief in 48 hours |
| Cost Considerations | Higher upfront cost, lower long-term expenses | Potential added costs for GI management |
Upgrade patient care with safer pain relief solutions!
As a trusted bulk manufacturer of transdermal patches and pain plasters, Enokon empowers healthcare distributors and brands with:
- Custom R&D: Tailor formulations to your patients’ needs.
- GI-Sparing Technology: Reduce adverse events linked to oral NSAIDs.
- Cost-Efficient Production: Scale high-quality patches without compromising safety.
Let’s discuss how transdermal delivery can elevate your formulary—contact our team for samples or partnership inquiries!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















