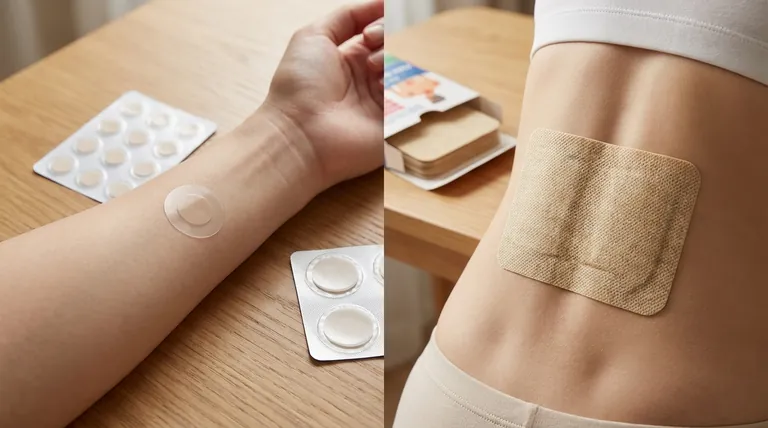
Transdermal patches and heat patches serve distinct purposes in medical and therapeutic applications. While both adhere to the skin, transdermal patches are designed to deliver active pharmaceutical ingredients into the bloodstream over time, offering systemic treatment for conditions like chronic pain or nicotine addiction. Heat patches, on the other hand, generate warmth through chemical reactions to provide localized pain relief without drug delivery. The differences extend to their mechanisms, design complexity, and regulatory classifications, making them suited for different clinical and consumer needs.
Key Points Explained:
-
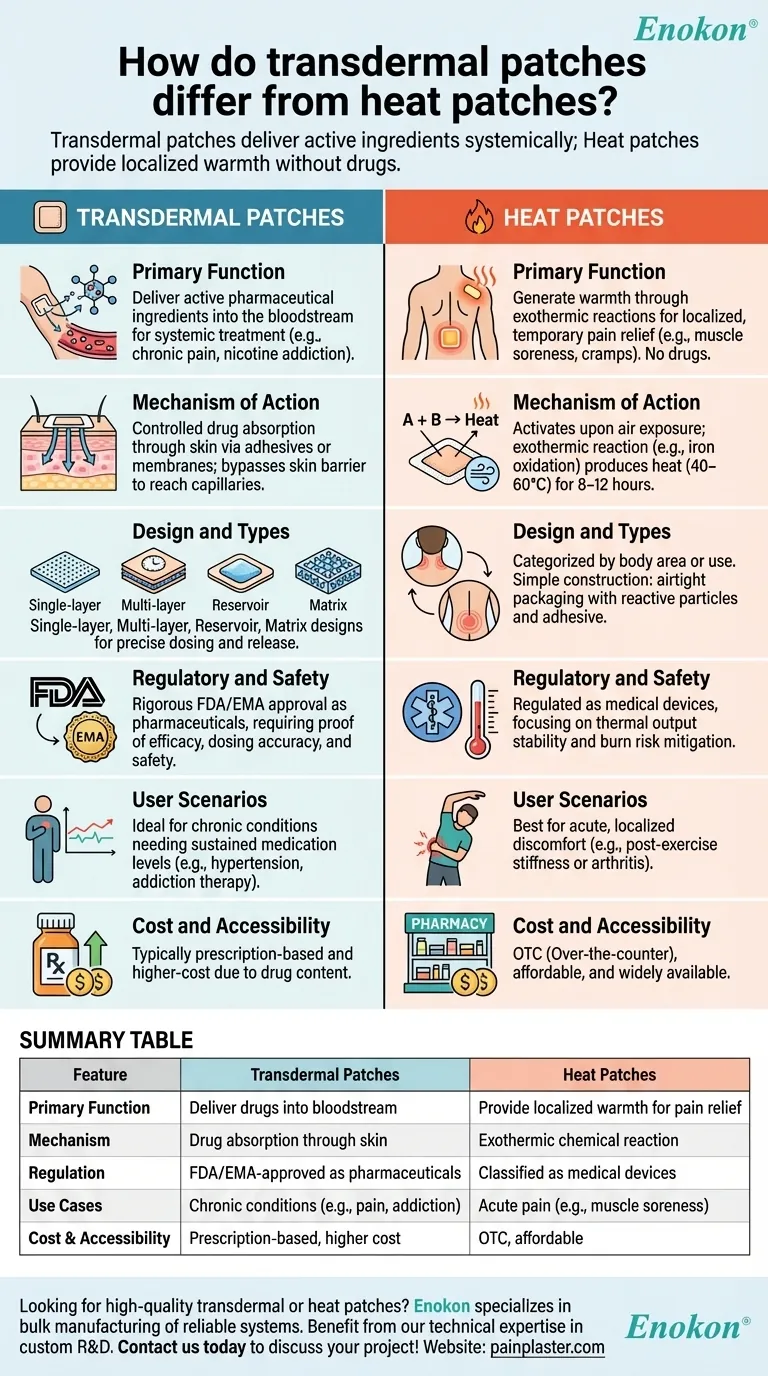
Primary Function
- Transdermal patches (transdermal patch) are drug delivery systems that release active ingredients (e.g., fentanyl, nicotine) through the skin into systemic circulation. They treat conditions requiring sustained medication levels.
- Heat patches rely on exothermic reactions (e.g., iron powder oxidation) to produce localized warmth for temporary pain relief (e.g., muscle soreness, menstrual cramps). They contain no drugs and are classified as medical devices rather than pharmaceuticals.
-
Mechanism of Action
-
Transdermal patches:
- Use permeation-enhancing adhesives or membranes to control drug absorption rates.
- Require precise formulation to bypass the skin barrier (stratum corneum) and reach capillaries.
-
Heat patches:
- Activate upon air exposure, generating heat (typically 40–60°C) for 8–12 hours.
- Provide vasodilation and muscle relaxation, but no pharmacological effect.
-
Transdermal patches:
-
Design and Types
-
Transdermal patches:
- Single-layer: Drug-adhesive matrix for low-dose delivery (e.g., nicotine patches).
- Multi-layer: Added control layers for timed release (e.g., opioid pain patches).
- Reservoir: Liquid/gel drug compartment with rate-limiting membranes (e.g., clonidine patches).
- Matrix: Polymer-based adhesive for uniform release (e.g., hormone therapy).
-
Heat patches:
- Categorized by body area (e.g., neck, lower back) or specific use (e.g., period pain).
- Simple construction: airtight packaging with reactive particles and adhesive backing.
-
Transdermal patches:
-
Regulatory and Safety Considerations
- Transdermal patches undergo rigorous FDA/EMA approval as drugs, requiring proof of efficacy, dosing accuracy, and safety.
- Heat patches are regulated as medical devices, focusing on thermal output stability and burn risk mitigation.
-
User Scenarios
- Transdermal patches: Ideal for chronic conditions needing steady drug levels (e.g., hypertension, addiction therapy).
- Heat patches: Best for acute, localized discomfort (e.g., post-exercise stiffness or arthritis).
-
Cost and Accessibility
- Transdermal patches are typically prescription-based and higher-cost due to drug content.
- Heat patches are OTC, affordable, and widely available in pharmacies.
Understanding these differences helps purchasers select the right product for clinical or retail needs, balancing therapeutic goals with safety and cost. Would the intended use be for managing long-term medication or immediate pain relief? This distinction guides procurement decisions.
Summary Table:
| Feature | Transdermal Patches | Heat Patches |
|---|---|---|
| Primary Function | Deliver drugs into bloodstream | Provide localized warmth for pain relief |
| Mechanism | Drug absorption through skin | Exothermic chemical reaction |
| Regulation | FDA/EMA-approved as pharmaceuticals | Classified as medical devices |
| Use Cases | Chronic conditions (e.g., pain, addiction) | Acute pain (e.g., muscle soreness) |
| Cost & Accessibility | Prescription-based, higher cost | OTC, affordable |
Looking for high-quality transdermal patches or heat patches for your healthcare or retail needs? Enokon specializes in bulk manufacturing of reliable transdermal drug delivery systems and pain relief patches. Benefit from our technical expertise in custom R&D and development to meet your specific requirements. Contact us today to discuss your project!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
















