To be clear, the high-concentration capsaicin 8% dermal patch is a clinically effective treatment for painful diabetic peripheral neuropathy. A single, 30-minute application has been shown to provide significant pain relief that can last for up to 12 weeks, while also improving sleep quality for patients.
The core takeaway is that the capsaicin 8% patch offers a fundamentally different approach to pain management. Instead of a daily systemic medication, it provides targeted, localized, and long-lasting relief from neuropathic pain with a lower risk of systemic side effects.
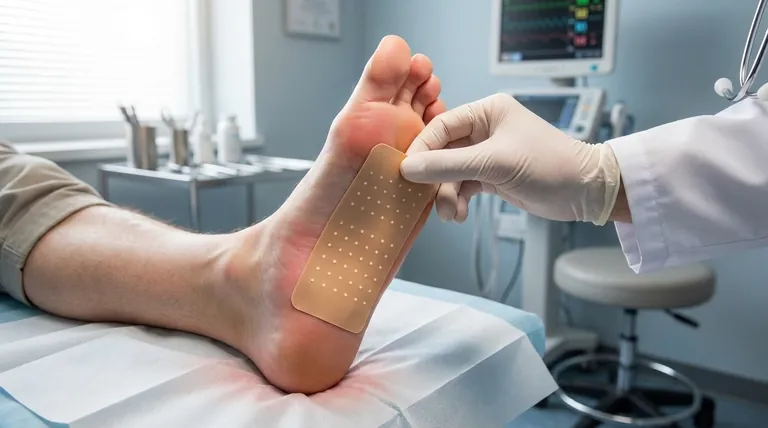
The Mechanism: How It Provides Lasting Relief
Targeting the Source of Pain
The capsaicin 8% patch is not a simple painkiller; it works by directly targeting the body's pain-sensing nerve fibers in the skin.
The high concentration of capsaicin overwhelms these nerve endings, causing them to become "defunctionalized" for an extended period. This effectively turns down the volume on the constant pain signals being sent to the brain.
A Long-Acting, Reversible Effect
The effect is long-lasting but not permanent. Over approximately three months, the nerve endings gradually recover their function, which is why the treatment is typically repeated on a quarterly basis.
The Treatment Experience: What to Expect
A Supervised, In-Office Procedure
Due to its high potency, the capsaicin 8% patch is applied by a healthcare professional in a clinical setting. Up to four patches may be used to cover the most painful areas, typically on the feet, for a duration of 30 minutes.
Onset and Duration of Relief
While some initial relief may be noticed sooner, studies show patients experience meaningful pain reduction around 19 days after the first application.
The key benefit is the duration of this effect. A single treatment provides sustained pain relief for 12 weeks, or about three months.
Long-Term Safety and Efficacy
For those requiring ongoing management, repeat treatments over a 52-week period have been shown to provide sustained pain relief. Importantly, these long-term studies found no evidence of negative neurological effects compared to standard care alone.
Understanding the Trade-offs
Advantage: Localized vs. Systemic Treatment
The primary advantage of the patch is its localized action. Unlike oral medications such as pregabalin or opioids, the active ingredient is not absorbed systemically in significant amounts.
This dramatically reduces the risk of common side effects like drowsiness, constipation, nausea, or cognitive fog, which are often associated with oral pain medications.
Common Side Effect: Application Site Reactions
The most common side effects are temporary and localized to the application site. Patients should expect to feel a sensation of burning, stinging, and redness during and shortly after treatment. These reactions are a normal part of the mechanism and typically resolve on their own.
Efficacy Can Vary by Condition
It is important to note that while highly effective for peripheral neuropathies like PDPN, its results can vary. For example, studies in patients with HIV-associated neuropathy have shown inconsistent or equivocal results, highlighting that it is not a universal solution for all types of nerve pain.
Comparison to Standard Oral Medication
Pain Relief and Patient Satisfaction
When compared directly to oral pregabalin for peripheral neuropathic pain, the capsaicin 8% patch was found to be non-inferior in its ability to relieve pain.
Crucially, the patch was associated with a faster onset of action and resulted in greater overall treatment satisfaction among patients.
Making the Right Choice for Your Goal
The capsaicin 8% patch is a powerful and useful addition to the available treatments for painful diabetic neuropathy. Deciding if it's right for you involves weighing its unique profile against your personal priorities.
- If your primary focus is minimizing systemic side effects: The patch is an excellent choice, as it works directly where you feel pain without affecting the rest of your body.
- If your primary focus is a convenient, low-maintenance schedule: A single, 30-minute in-office treatment every three months may be preferable to taking a pill one or more times every day.
- If your primary focus is sustained relief without daily medication: The patch is specifically designed to provide a long-acting effect from one application, freeing you from the burden of daily pain management.
This treatment offers a valuable, targeted tool for reclaiming quality of life from chronic diabetic nerve pain.
Summary Table:
| Key Attribute | Details |
|---|---|
| Primary Use | Treatment for Painful Diabetic Peripheral Neuropathy (PDPN) |
| Mechanism | Defunctionalizes pain-sensing nerve fibers in the skin for long-lasting relief |
| Application | Single, 30-minute, in-office procedure by a healthcare professional |
| Onset of Relief | Meaningful pain reduction typically seen around 19 days post-application |
| Duration of Effect | Sustained pain relief for up to 12 weeks (approximately 3 months) |
| Key Advantage | Localized action minimizes systemic side effects (e.g., drowsiness, nausea) |
| Common Side Effects | Temporary application site reactions (burning, stinging, redness) |
Empower Your Patients with Targeted Pain Relief
As a healthcare distributor or brand, offering advanced, effective treatments is key to your success. The capsaicin 8% patch represents a significant opportunity in the growing market for non-opioid pain management.
Partner with Enokon to:
- Supply Reliable Patches: We are a bulk manufacturer of high-quality, reliable transdermal patches and pain plasters.
- Leverage Custom R&D: Benefit from our deep technical expertise for custom formulation and development to meet your specific market needs.
- Enhance Your Portfolio: Provide your customers with a clinically proven, long-acting solution that improves patient satisfaction and quality of life.
Let's discuss how we can support your goals. Contact our experts today to explore partnership opportunities and supply chain solutions.
Visual Guide

Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
















