To be included in this specific review, studies had to be English-language articles published in the MEDLINE database on or before November 2010. The research must have described the development, use, safety, or efficacy of licensed transdermal patch treatments for neurologic conditions specifically affecting elderly patients.
The selection criteria for a scientific review are deliberately narrow. They are designed to isolate a very specific question—in this case, the use of approved patches for older adults with neurological issues—to ensure that the findings are consistent and directly comparable.

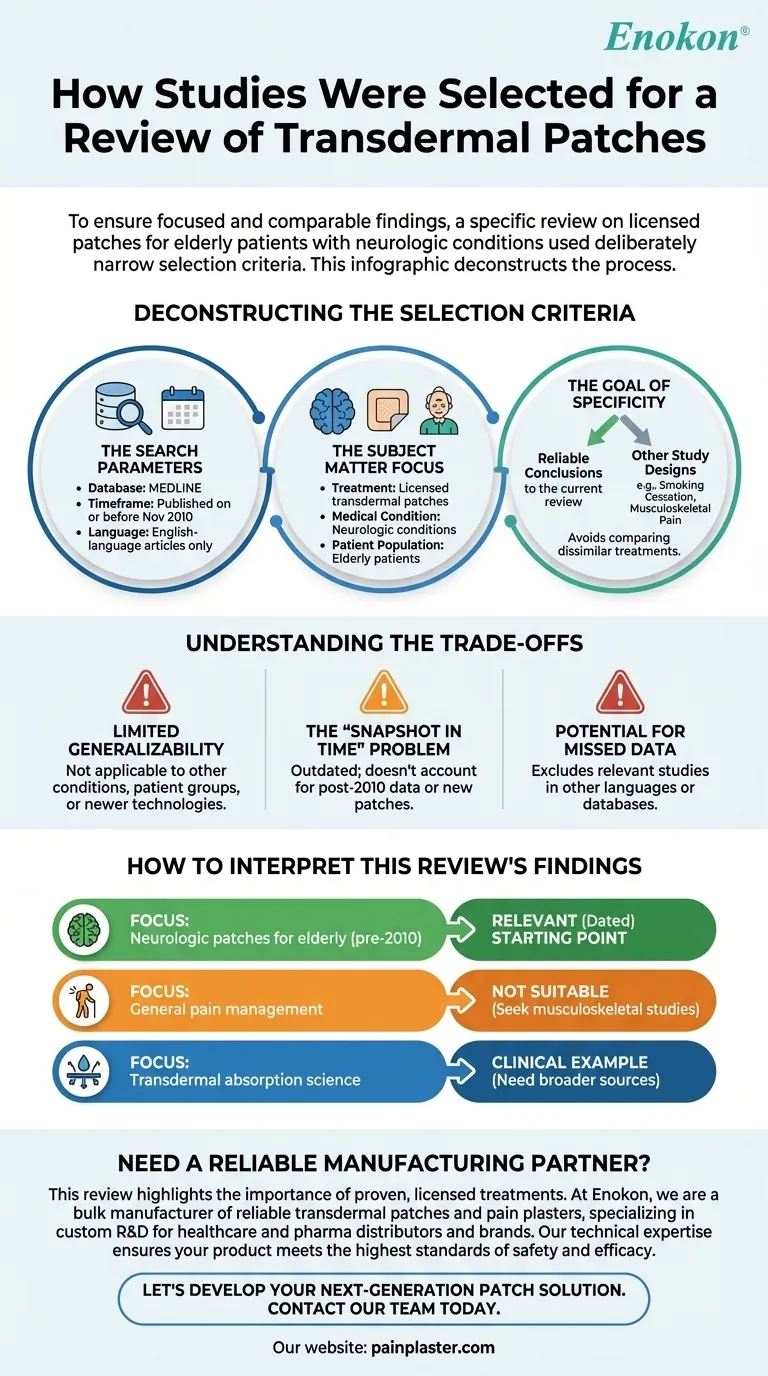
Deconstructing the Selection Criteria
To understand the scope and limitations of the review, it's essential to break down each component of its selection process. Each criterion acts as a filter, shaping the final pool of evidence.
The Search Parameters
The initial search was defined by three key constraints: the database, the date, and the language.
- Database: The search was limited to MEDLINE, a major and reputable database for biomedical literature.
- Timeframe: The search was conducted in November 2010, meaning any research published after this date was not included.
- Language: Only English-language articles were considered, a common but important limitation that can exclude relevant international research.
The Subject Matter Focus
The review focused on a very precise intersection of treatment type, medical condition, and patient population.
- Treatment Type: It only included studies on licensed transdermal patches, meaning treatments that have received regulatory approval for use.
- Medical Condition: The scope was restricted to neurologic conditions, such as those affecting the brain, spinal cord, and nerves.
- Patient Population: The review specifically targeted studies involving elderly patients, a group with unique physiological considerations.
How Selection Criteria Shape a Review's Conclusion
The purpose of strict inclusion criteria is to create a focused, high-quality analysis. Different research questions demand different criteria.
The Goal of Specificity
By narrowing the focus to licensed patches for a specific condition in a defined age group, researchers can draw more reliable conclusions for that exact scenario. This avoids comparing dissimilar treatments or patient outcomes.
Contrasting with Other Study Designs
Other reviews mentioned in the references highlight this principle. A review on smoking cessation patches required studies to be randomized, double-blind trials of at least 12 weeks. Another analysis on pain relief looked at musculoskeletal conditions, a completely different clinical area.
Each set of criteria is tailored to answer a different clinical question effectively.
Understanding the Trade-offs
This highly specific approach has inherent limitations that are critical for interpreting the findings objectively.
Limited Generalizability
The conclusions drawn from this review are only directly applicable to the use of licensed neurologic patches in the elderly as of 2010. They cannot be generalized to other conditions (like musculoskeletal pain), other patient groups, or newer patch technologies.
The "Snapshot in Time" Problem
Because the search was performed in 2010, the review is now more than a decade old. It does not account for any new patches, safety data, or efficacy studies that have emerged since.
Potential for Missed Data
Relying on a single database and only English-language articles means that relevant studies published elsewhere or in other languages would have been missed, potentially biasing the results.
How to Interpret This Review's Findings
Understanding the selection criteria allows you to determine how to apply the information to your own goals.
- If your primary focus is on neurologic patches for the elderly before 2010: This review is a highly relevant, though dated, starting point for your research.
- If your primary focus is on general pain management: This review is not suitable; you should seek out research specifically on anti-inflammatory patches for musculoskeletal conditions.
- If your primary focus is on the science of transdermal absorption: This review offers a clinical application example, but you will need broader sources that discuss factors like molecular size and fat-solubility.
Ultimately, evaluating how studies were selected is the first step in understanding the true meaning and limitations of any scientific review.
Summary Table:
| Criterion | Requirement |
|---|---|
| Database | MEDLINE |
| Publication Date | On or before November 2010 |
| Language | English |
| Treatment Type | Licensed transdermal patches |
| Medical Condition | Neurologic conditions |
| Patient Population | Elderly patients |
Need a reliable manufacturing partner for your transdermal patch project?
This review highlights the importance of using proven, licensed treatments. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters, specializing in custom R&D for healthcare and pharma distributors and brands. Our technical expertise ensures your product meets the highest standards of safety and efficacy.
Let's develop your next-generation patch solution. Contact our team today to discuss your requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
















