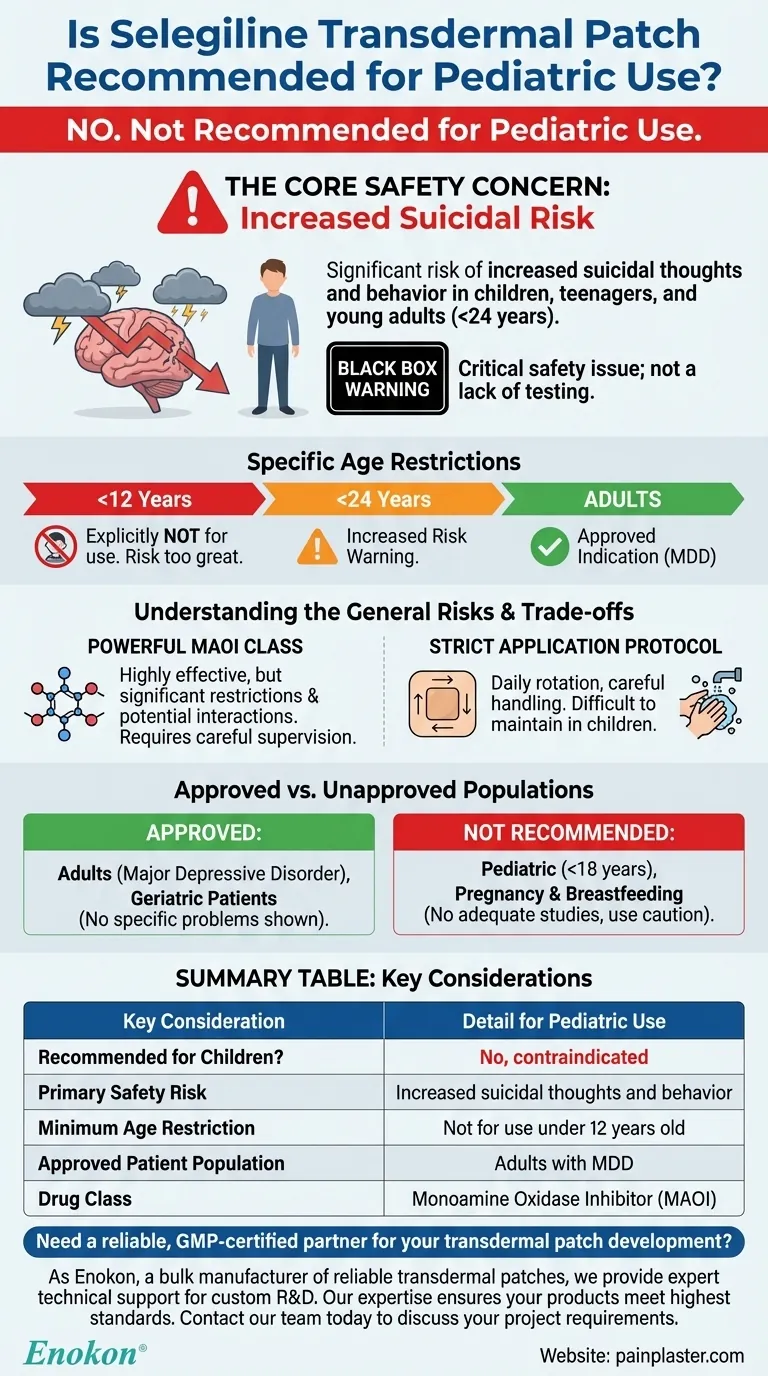
No, the selegiline transdermal patch is not recommended for pediatric use. Clinical studies have demonstrated a significant risk, showing that some children, teenagers, and young adults taking this medicine may experience an increase in suicidal thoughts or attempts. For this reason, it is explicitly not to be used in children younger than 12 years of age.
The core takeaway is that the selegiline transdermal patch carries a serious safety warning regarding an increased risk of suicidal ideation in younger populations, making it unsuitable and unapproved for treating depression in children.

The Core Safety Concern: Increased Suicidal Risk
The primary reason selegiline is not used in pediatric patients is a well-documented and critical safety issue. This is not a matter of a lack of testing; it is a known adverse effect.
The Black Box Warning
Antidepressants, including selegiline, have been linked to an increased risk of suicidal thinking and behavior in patients under the age of 24. This is a serious warning that healthcare providers and families must consider when evaluating treatment options for depression in this age group.
Specific Age Restrictions
Medical guidance is definitive on this point: the selegiline transdermal patch should not be used in any child younger than 12 years of age. The risk is considered too great to justify its potential benefits in this vulnerable population.
A Powerful Class of Medication
Selegiline is a monoamine oxidase inhibitor (MAOI). This class of drugs is highly effective but also comes with significant restrictions and potential interactions, requiring careful medical supervision even in its intended adult population.
Approved Use and Other Populations
To understand why the pediatric restriction is so firm, it helps to see where the medication is approved for use and where caution is still advised.
Primary Indication: Adults with Depression
The selegiline transdermal patch is a prescription medication specifically used to treat major depressive disorder in adults. Its safety and efficacy have been established for this group.
Use in Geriatric Patients
In contrast to the pediatric population, appropriate studies have not shown any geriatric-specific problems that would limit the usefulness of the selegiline patch in the elderly.
Use During Pregnancy and Breastfeeding
There are no adequate studies to determine the risk to an infant when this medication is used during breastfeeding. This highlights a general principle: potent medications are used with extreme caution or avoided entirely when a vulnerable population (like an infant or child) could be affected.
Understanding the General Risks and Trade-offs
The stringent handling and monitoring requirements for selegiline underscore why it is not appropriate for children. Careful management is essential for any patient.
Strict Application Protocol
The patch must be applied once daily to clean, dry skin, and the application site must be rotated each day. It cannot be cut, must be replaced if it falls off, and requires careful handling, including washing hands after application. This strict protocol can be challenging to maintain consistently in pediatric patients.
Critical Medical Disclosures
Before use, a doctor must be informed of numerous conditions, including allergies, pheochromocytoma (a type of tumor), seizures, or heart disease. The potential for serious drug interactions means all other medications must be disclosed.
Potential for Impairment
The patch may cause drowsiness or dizziness. This requires patients to avoid driving or operating heavy machinery until they understand how the medication affects them, a precaution that is difficult to manage with children.
Making the Right Choice for Your Goal
When considering treatment for depression, the patient's age is a critical factor in determining a safe and appropriate course of action.
- If your primary focus is treating a child or adolescent (<18 years): The selegiline transdermal patch is not a safe or recommended option due to the established risk of increased suicidal thoughts.
- If your primary focus is treating an adult with depression: This medication is an approved option, but it requires strict adherence to your doctor's instructions and full disclosure of your medical history to be used safely.
Ultimately, any decision regarding mental health treatment for a child must be made in close consultation with a qualified pediatric healthcare professional.
Summary Table:
| Key Consideration | Detail for Pediatric Use |
|---|---|
| Recommended for Children? | No, contraindicated |
| Primary Safety Risk | Increased suicidal thoughts and behavior |
| Minimum Age Restriction | Not for use in children under 12 years old |
| Approved Patient Population | Adults with major depressive disorder |
| Drug Class | Monoamine Oxidase Inhibitor (MAOI) |
Need a reliable, GMP-certified partner for your transdermal patch development? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide expert technical support for custom R&D and development. Our expertise ensures your products meet the highest safety and efficacy standards. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















