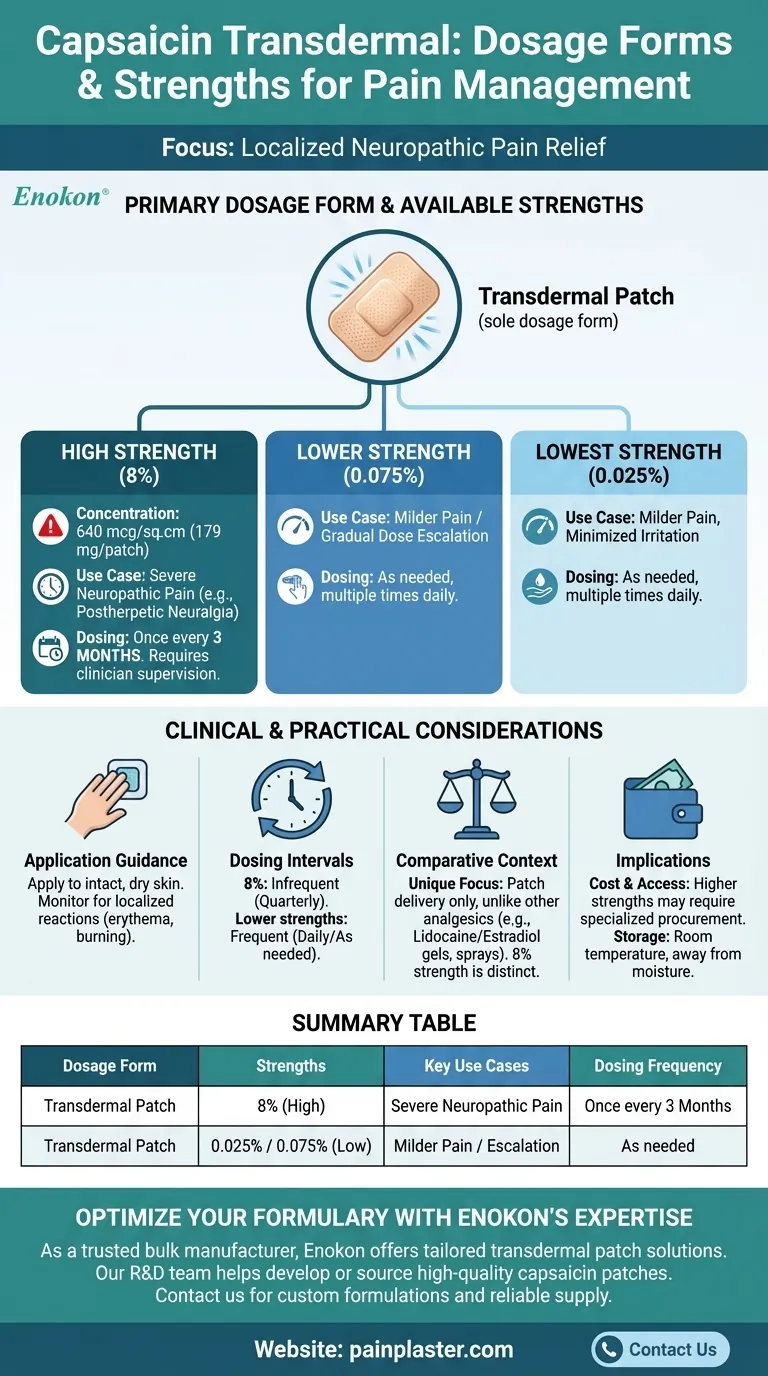
Capsaicin transdermal is primarily available as a transdermal patch, designed for localized pain relief by delivering capsaicin directly through the skin. The available strengths include 8% (containing 640 mcg/sq.cm or 179 mg/patch), 0.025%, and 0.075%. These concentrations cater to varying pain intensities and treatment requirements, offering flexibility in dosing for conditions like neuropathic pain. The Capsaicin Transdermal Patch is a key option for patients seeking non-systemic pain management solutions.

Key Points Explained:
-
Dosage Forms
- Transdermal Patch: The sole dosage form for capsaicin transdermal, ensuring controlled and sustained release. Patches are applied directly to the skin, targeting localized pain areas without systemic absorption.
-
Available Strengths
- 8% Strength: Contains 640 mcg/sq.cm or 179 mg per patch. This high concentration is typically used for severe neuropathic pain, such as postherpetic neuralgia.
- 0.025% and 0.075% Strengths: Lower concentrations suitable for milder pain or patients requiring gradual dose escalation. These options minimize irritation while maintaining efficacy.
-
Clinical Considerations
- Patient Selection: Higher strengths (e.g., 8%) are reserved for patients unresponsive to lower doses or with significant pain.
- Application Guidance: Patches should be applied to intact, dry skin and monitored for localized reactions like erythema or burning.
- Dosing Intervals: Typically applied once every 3 months (for 8%) or as needed for lower strengths, aligning with chronic pain management protocols.
-
Comparative Context
- Unlike transdermal estradiol or lidocaine (which offer gels/sprays or multiple patch strengths), capsaicin transdermal focuses solely on patch delivery, emphasizing its niche in neuropathic pain.
- The 8% patch’s unique high concentration distinguishes it from other transdermal analgesics, requiring clinician supervision for safe use.
-
Practical Implications
- Cost and Accessibility: Higher strengths may involve specialized procurement or insurance pre-authorization.
- Storage: Patches should be stored at room temperature, away from moisture, to maintain stability.
For purchasers, understanding these variations ensures alignment with clinical needs, formulary requirements, and patient tolerance profiles. Have you considered how the 8% patch’s infrequent dosing might reduce long-term costs despite its higher unit price? This balance of efficacy and convenience underscores its role in modern pain management.
Summary Table:
| Dosage Form | Strengths | Key Use Cases |
|---|---|---|
| Transdermal Patch | 8% (640 mcg/sq.cm, 179 mg) | Severe neuropathic pain (e.g., postherpetic neuralgia) |
| Transdermal Patch | 0.025%, 0.075% | Milder pain or gradual dose escalation |
| Clinical Notes | Application | Dosing Frequency |
|---|---|---|
| High-strength (8%) requires supervision | Apply to intact, dry skin | Once every 3 months (8%) or as needed (lower strengths) |
Optimize your pain management formulary with Enokon’s expertise
As a trusted bulk manufacturer of transdermal patches, Enokon offers tailored solutions for healthcare distributors and brands. Our technical R&D team can help you develop or source high-quality capsaicin patches in varying strengths to meet clinical and market demands.
Contact us today to discuss custom formulations, competitive pricing, and reliable supply chains for your pain relief products.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief

















