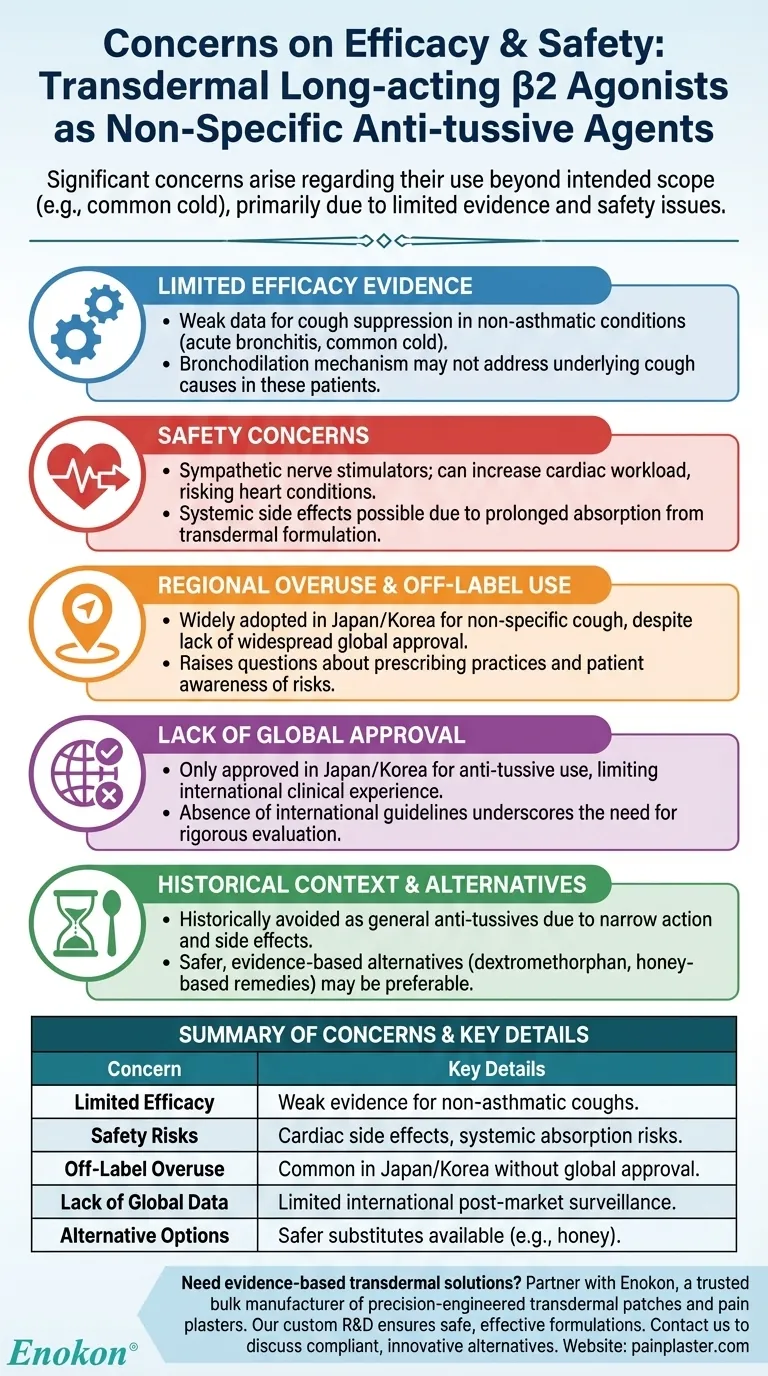
The use of transdermal long-acting β2 agonists as non-specific anti-tussive agents raises significant concerns regarding efficacy and safety. Primarily used in Japan and Korea, these agents are often employed beyond their intended scope (e.g., for common colds), despite limited evidence supporting their effectiveness. Safety issues, particularly cardiac side effects due to their sympathetic nerve-stimulating properties, further complicate their use. The lack of robust clinical data and regional approval restrictions highlight the need for cautious evaluation before widespread adoption.
Key Points Explained:
-
Limited Evidence for Efficacy
- Current data does not strongly support the anti-tussive effectiveness of transdermal β2 agonists, especially for non-asthmatic conditions like acute bronchitis or the common cold.
- Their mechanism of action (bronchodilation) may not directly address the underlying causes of cough in non-asthmatic patients.
-
Safety Concerns
- As sympathetic nerve stimulators, β2 agonists can increase cardiac workload, posing risks for individuals with pre-existing heart conditions.
- Transdermal formulations, while bypassing first-pass metabolism, may still lead to systemic side effects due to prolonged absorption.
-
Regional Overuse and Off-Label Use
- In Japan, these patches are widely adopted for non-specific cough suppression, despite lacking approval for such indications in most countries.
- This over-adherence raises questions about prescribing practices and patient awareness of potential risks.
-
Lack of Global Approval
- Only Japan and Korea have approved transdermal β2 agonists for anti-tussive use, limiting global clinical experience and post-market surveillance data.
- The absence of international guidelines or consensus further underscores the need for rigorous evaluation.
-
Historical Context and Alternatives
- Before transdermal formulations, β2 agonists were avoided as general anti-tussives due to their narrow site of action and side effect profile.
- Safer, evidence-based alternatives (e.g., dextromethorphan, honey-based remedies) may be preferable for non-asthmatic cough.
For healthcare providers, balancing patient demand with evidence-based practice is critical. Have you considered how regional prescribing trends might influence global perceptions of this therapy? The cautious approach aligns with technologies that quietly shape modern healthcare—weighing innovation against patient safety.
Summary Table:
| Concern | Key Details |
|---|---|
| Limited Efficacy | Weak evidence for cough suppression in non-asthmatic conditions (e.g., colds). |
| Safety Risks | Cardiac side effects due to sympathetic stimulation; systemic absorption risks. |
| Off-Label Overuse | Common in Japan/Korea despite lack of global approval for cough suppression. |
| Lack of Global Data | Limited post-market surveillance outside approved regions. |
| Alternative Options | Safer substitutes (e.g., dextromethorphan, honey) available. |
Need evidence-based transdermal solutions? Partner with Enokon, a trusted bulk manufacturer of precision-engineered transdermal patches and pain plasters for healthcare distributors and brands. Our expertise in custom R&D ensures safe, effective formulations tailored to your needs. Contact our team to discuss compliant, innovative alternatives.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What precautions should be taken when using pain relief patches? Essential Safety Guide
- What was the reported pain relief after the initial month of plaster use? Consistent & Effective Pain Management
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management














