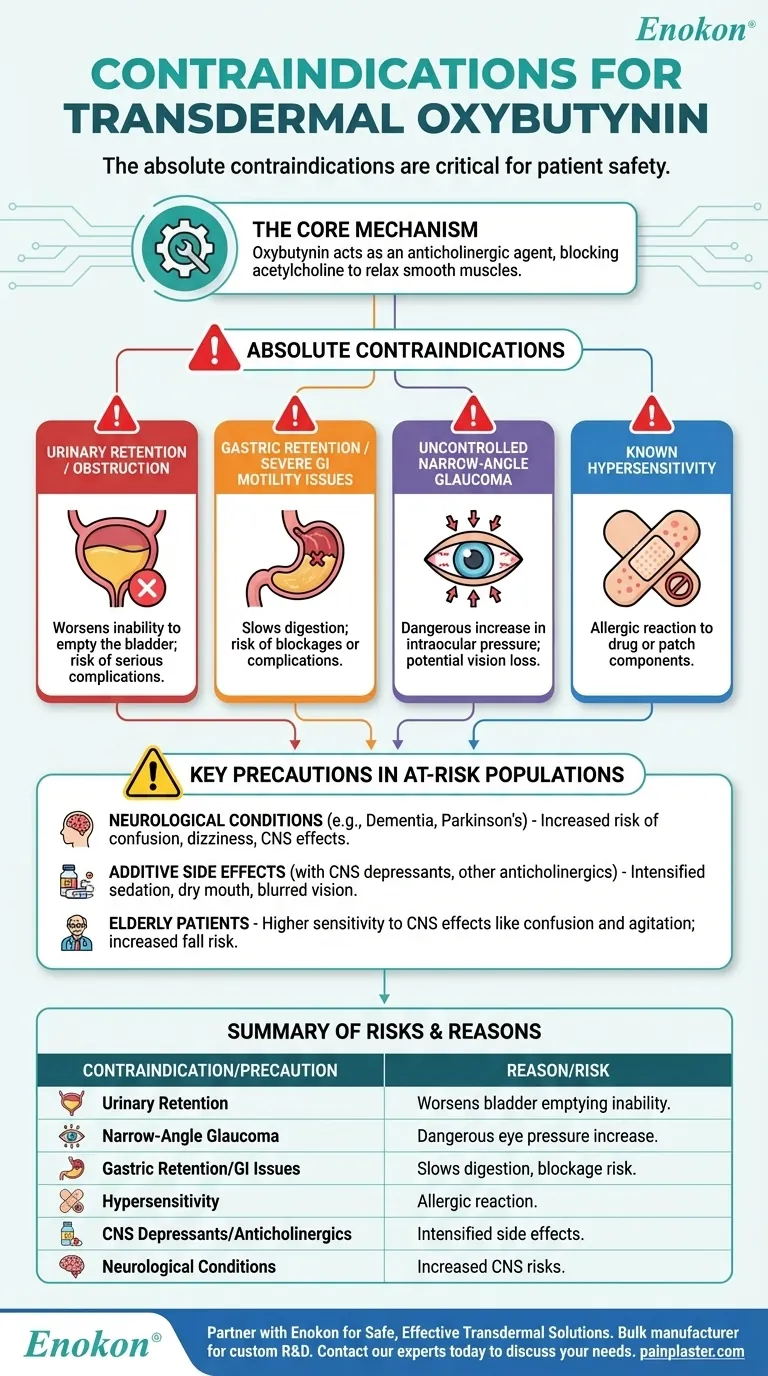
The absolute contraindications for transdermal oxybutynin are critical for patient safety. This medication should not be used in patients with a known hypersensitivity to the drug, urinary retention, gastric retention, severely decreased gastrointestinal motility, or uncontrolled narrow-angle glaucoma. These conditions are directly worsened by the drug's mechanism of action.
Oxybutynin's primary function is to relax smooth muscles by blocking a neurotransmitter called acetylcholine. Consequently, its contraindications are all conditions where further muscle relaxation in the urinary, digestive, or ocular systems would be dangerous.

The Core Mechanism: Why These Contraindications Exist
To understand the risks, it's essential to understand how oxybutynin works. As an anticholinergic agent, it blocks the action of acetylcholine, a chemical messenger that triggers muscle contractions in various parts of the body.
The Impact on the Urinary System
Oxybutynin is prescribed to treat overactive bladder by relaxing the bladder muscle, which reduces the urge to urinate.
However, if a patient already has urinary retention or a bladder outflow obstruction, the drug will worsen the inability to empty the bladder. This can lead to serious complications.
The Impact on the Gastrointestinal System
The same muscle-relaxing effect that helps the bladder also slows down the contractions of the stomach and intestines.
For individuals with gastric retention or severe gastrointestinal motility issues, this can be dangerous. The medication can cause food to remain in the stomach or slow its passage through the intestines, leading to blockages or other complications.
The Impact on the Eyes
Anticholinergic drugs can cause the pupil to dilate, which in susceptible individuals can narrow the angle where fluid drains from the eye.
In a patient with uncontrolled narrow-angle glaucoma, this can cause a sudden and dangerous increase in intraocular pressure, potentially leading to vision loss.
Key Precautions in At-Risk Populations
Beyond absolute contraindications, extreme caution is necessary for certain patients who may be more vulnerable to oxybutynin's side effects.
Patients with Neurological Conditions
Individuals with conditions like myasthenia gravis, dementia, Parkinson's disease, or autonomic neuropathy should use oxybutynin cautiously. The drug can cross into the brain and cause or worsen CNS side effects like dizziness, drowsiness, and confusion.
Risk of Additive Side Effects
Taking oxybutynin with other anticholinergic drugs will intensify side effects like severe dry mouth, constipation, and blurred vision.
Similarly, using it with CNS depressants, including alcohol or medications that cause drowsiness, can lead to profound sedation and cognitive impairment.
Application Site Reactions
As a transdermal patch, oxybutynin can cause local skin irritation. More importantly, any patient with a known hypersensitivity or allergy to the drug or patch components must avoid it entirely.
Understanding the Trade-offs and Risks
The primary risk of oxybutynin is not that it will create a new disease, but that it will severely exacerbate an existing, underlying medical condition.
Worsening of Existing Conditions
The core danger is using the drug when a contraindication is present but undiagnosed. A patient's complete medical history is the most critical screening tool for ensuring this medication's safe use.
Central Nervous System (CNS) Effects
The potential for confusion, dizziness, and agitation is a significant concern, especially in the elderly population. These side effects can increase the risk of falls and decrease a person's quality of life.
Overdose Symptoms
In the case of an overdose (for instance, if multiple patches are applied), the drug's effects become more pronounced. Symptoms may include agitation, a fast or irregular heartbeat, and severe confusion, requiring immediate medical attention.
Making a Safe Decision
Your medical history is the definitive guide to whether transdermal oxybutynin is a safe choice for you.
- If your primary focus is treating overactive bladder but you have a history of urinary issues: You must ensure your doctor fully understands your condition, as oxybutynin can prevent proper bladder emptying.
- If you have any gastrointestinal motility disorders like GERD or gastroparesis: This medication is likely unsafe as it can further slow your digestive system, leading to serious complications.
- If you have been diagnosed with glaucoma: It is essential to confirm with an ophthalmologist that it is not "uncontrolled narrow-angle" glaucoma before considering this drug.
- If you care for an elderly individual: Be vigilant for new or worsening confusion, dizziness, or drowsiness, as they are particularly sensitive to the CNS effects.
A thorough review of your medical history with your healthcare provider is the most important step in using oxybutynin transdermal safely and effectively.
Summary Table:
| Contraindication / Precaution | Reason / Risk |
|---|---|
| Urinary Retention | Worsens inability to empty the bladder. |
| Uncontrolled Narrow-Angle Glaucoma | Can cause dangerous increase in eye pressure. |
| Gastric Retention / Severe GI Motility Issues | Slows digestion, risk of blockages. |
| Known Hypersensitivity | Allergic reaction to drug or patch components. |
| Use with CNS Depressants / Other Anticholinergics | Intensified side effects like sedation and dry mouth. |
| Patients with Neurological Conditions (e.g., Dementia) | Increased risk of confusion, dizziness. |
Partner with Enokon for Safe, Effective Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with the technical expertise needed for custom R&D and development. Ensure your products are formulated with patient safety as the top priority.
Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief















