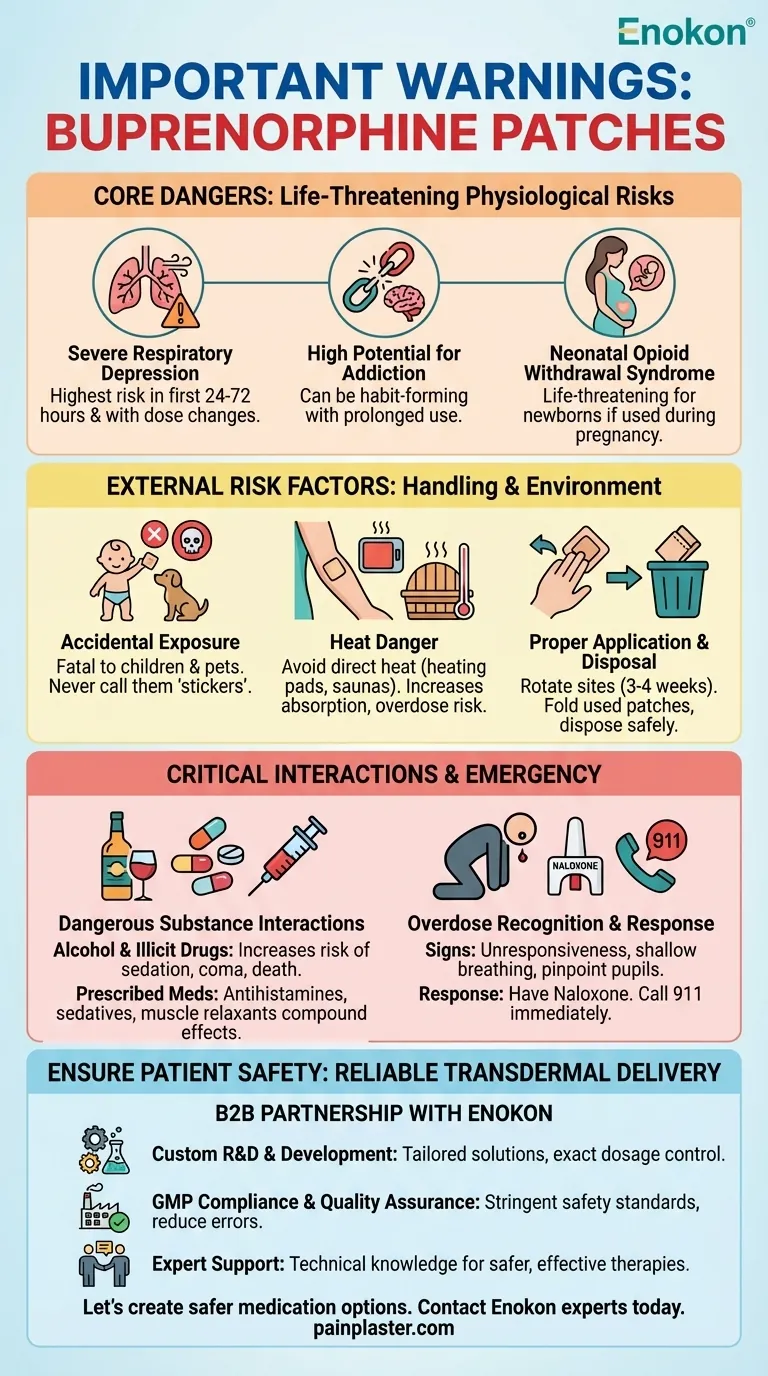
The primary warnings for buprenorphine patches center on the profound risk of severe, life-threatening respiratory depression (breathing problems). This danger is significantly amplified by interactions with other substances, incorrect application, or accidental exposure. Other critical warnings involve the high potential for addiction, the risk of causing withdrawal symptoms in newborns, and the danger of overdose from environmental factors like heat.
Buprenorphine is a powerful opioid, and its delivery via a patch introduces unique risks alongside standard ones. The most severe dangers arise not from the drug in isolation, but from its interaction with other depressants, environmental factors that increase absorption, and accidental exposure to non-prescribed individuals.
The Core Dangers: Understanding the Life-Threatening Risks
The most serious warnings are related to the direct physiological effects of buprenorphine, a potent opioid medication.
Severe Respiratory Depression
This is the most acute and life-threatening risk associated with buprenorphine. The body’s natural drive to breathe can be dangerously suppressed.
This risk is highest during the first 24 to 72 hours of starting treatment or after a dose increase, as your body adjusts to the medication.
High Potential for Addiction
Like other opioids, buprenorphine patches can be habit-forming, especially with prolonged use. Physical and psychological dependence can develop, leading to a risk of misuse.
Neonatal Opioid Withdrawal Syndrome
If used during pregnancy, the patch can cause the baby to be born with life-threatening withdrawal symptoms. This condition requires immediate medical intervention for the newborn.
Environmental and External Risk Factors
How you use and handle the patch is just as critical as the medication itself. External factors can turn a therapeutic dose into a dangerous one.
The Danger of Accidental Exposure
A buprenorphine patch can cause serious harm or death if it accidentally adheres to someone for whom it was not prescribed, especially a child.
Even used patches contain enough medication to be fatal to a child or pet. Never underestimate the potency of a discarded patch.
To prevent accidental exposure, never refer to the patches as "stickers" or "tattoos" in front of children.
The Critical Role of Heat
Avoid exposing the patch application site to direct heat sources like heating pads, saunas, electric blankets, or hot baths.
Heat increases the rate at which the medication is absorbed into your body, which can lead to a sudden, dangerous spike in drug levels and cause an overdose.
Proper Application and Disposal
Always apply the patch to a clean, dry, and non-irritated area of skin, such as the upper chest or outer arm. Change the application site with each new patch, and do not use the same spot for at least 3-4 weeks.
If a patch falls off, do not reapply it. Dispose of it safely and apply a new patch to a different location.
Used patches must be folded with the sticky sides together and returned to a pharmacy or disposed of according to specific instructions to prevent accidental exposure.
Understanding the Trade-offs: Critical Drug and Substance Interactions
Buprenorphine’s effects are drastically altered when combined with other substances. Full disclosure to your healthcare provider is essential for safety.
Combining with Alcohol or Illicit Drugs
Consuming alcohol or using street drugs while on buprenorphine patch therapy dramatically increases the risk of severe sedation, respiratory depression, coma, and death.
Prescribed Medication Interactions
A wide range of medications can interact dangerously with buprenorphine. These include, but are not limited to, antihistamines, sedatives, muscle relaxants, certain antidepressants, and other narcotics.
Taking these medications together can compound the sedative and respiratory-depressant effects, leading to a potential overdose.
The Importance of Full Medical History
Before starting treatment, you must inform your doctor of your entire medical history. Conditions like lung disease, liver problems, head injuries, or a history of addiction can significantly change how your body handles the medication and increase your risk of severe side effects.
Preparing for an Emergency: Overdose Recognition and Response
Knowing how to react in a potential overdose situation is a critical component of safe buprenorphine use.
Recognizing the Signs of Overdose
Key signs of an overdose include difficulty breathing or shallow breathing, extreme drowsiness leading to unresponsiveness, and pinpoint pupils.
The Role of Naloxone
It is strongly recommended to have the overdose-reversal medication naloxone available. Family members or caregivers should know where it is and how to use it.
Always call 911 or emergency services immediately after administering naloxone, as its effects are temporary and medical attention is still required.
Key Principles for Safe Use
Your approach to using this medication determines its safety and effectiveness.
- If your primary focus is preventing overdose: Avoid all other central nervous system depressants, especially alcohol, and be extremely cautious about exposing the patch to heat.
- If your primary focus is protecting others: Implement a strict storage and disposal protocol, ensuring children and pets can never access new or used patches.
- If your primary focus is ensuring treatment efficacy: Apply the patch exactly as directed, rotate application sites, and maintain open communication with your healthcare provider about all medications and side effects.
Informed and highly cautious use is the only way to manage the significant risks associated with buprenorphine patch therapy.
Summary Table:
| Warning Category | Key Risk | Key Prevention Action |
|---|---|---|
| Physiological Effects | Severe respiratory depression, addiction, neonatal withdrawal syndrome. | Monitor breathing, especially in first 72 hours. Inform doctor of pregnancy. |
| Environmental Factors | Accidental exposure (especially to children), overdose from heat (saunas, heating pads). | Store patches securely. Dispose of used patches properly. Avoid direct heat on patch. |
| Drug Interactions | Dangerous interactions with alcohol, sedatives, and other CNS depressants. | Disclose all medications and substances to your doctor. Avoid alcohol. |
| Overdose Response | Unresponsiveness, shallow breathing. | Have naloxone available. Call 911 immediately if overdose is suspected. |
Ensure Patient Safety with Reliable Transdermal Delivery
Buprenorphine patches require the highest standards of manufacturing consistency and safety to mitigate the severe risks outlined above. As a bulk manufacturer of reliable transdermal patches, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for precise, controlled drug delivery.
Partner with us to benefit from:
- Custom R&D and Development: Tailor your transdermal solutions, like pain plasters, with exact dosage control to minimize risks.
- GMP Compliance & Quality Assurance: Ensure every patch meets stringent safety standards, reducing the potential for application errors or inconsistent dosing.
- Expert Support: Leverage our deep technical knowledge to develop safer, more effective transdermal therapies for your patients.
Let's work together to create safer medication options. Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief














