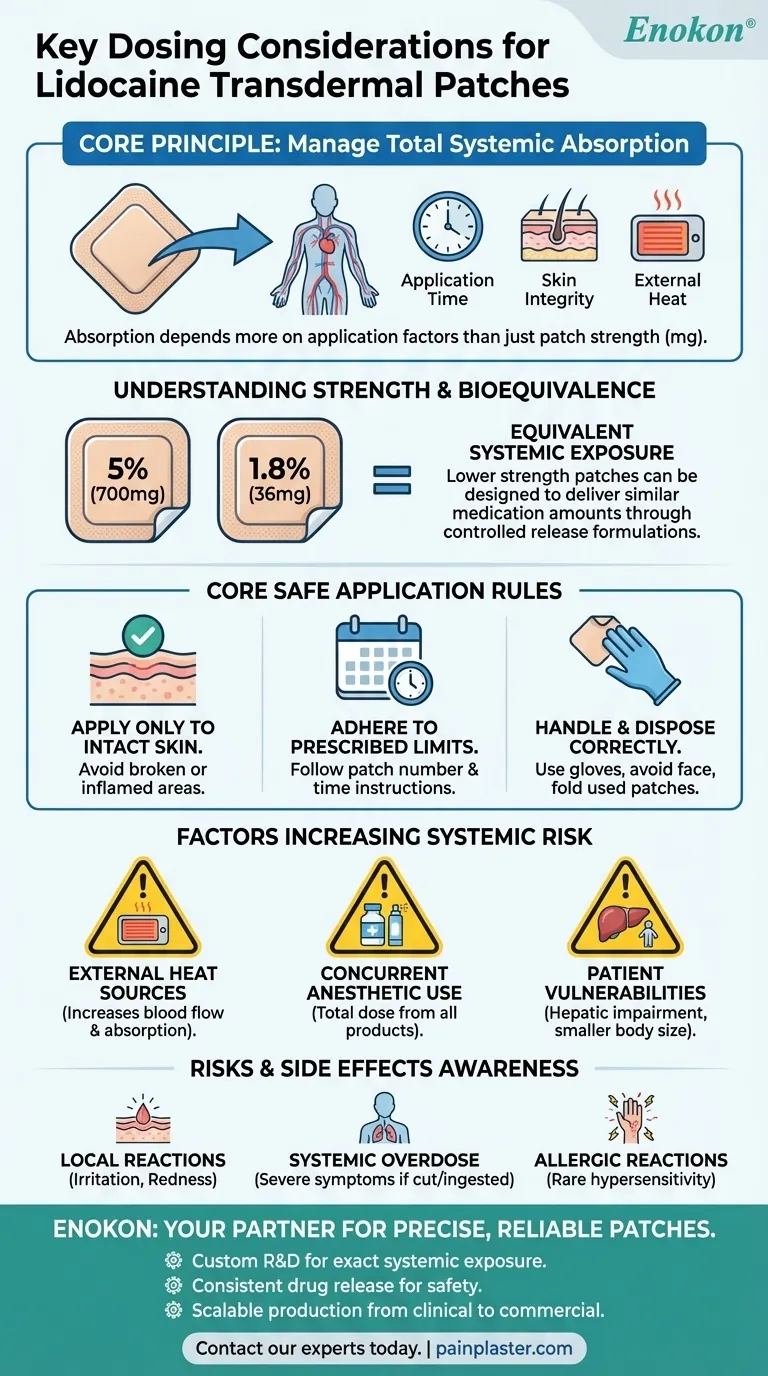
The most critical dosing consideration for lidocaine transdermal patches is managing total systemic absorption, not just the strength listed on the patch. Safe use requires applying the patch only to intact skin, adhering strictly to the recommended number of patches and application time, and accounting for any other local anesthetic products being used concurrently.
The core principle is that factors like application time, skin integrity, and external heat have a greater impact on the amount of lidocaine entering your bloodstream than the total milligrams of drug contained within the patch itself.
Understanding Patch Strength vs. Systemic Exposure
The amount of lidocaine in a patch can be misleading if viewed in isolation. The formulation of the patch controls the rate at which the drug is released into the skin, which is the key factor for both efficacy and safety.
Available Strengths
Lidocaine transdermal patches are commonly available in several strengths, including 5% (700mg per patch), 4%, and 1.8% (36mg per patch).
The Concept of Bioequivalence
It's crucial to understand that a lower-strength patch can be designed to deliver a similar amount of medication into the body as a higher-strength one. For instance, a 1.8% patch can provide an equivalent systemic exposure to a 5% patch due to differences in its adhesive and formulation that control drug release.
Core Principles of Safe Application
How and where you apply the patch directly influences its safety and effectiveness. Following these rules minimizes the risk of excessive absorption and potential toxicity.
Apply Only to Intact Skin
A patch must only be applied to healthy, unbroken skin. Applying it to broken, cut, or inflamed skin can dramatically increase the rate and amount of lidocaine absorbed into the bloodstream.
Adhere to Prescribed Limits
Never use more patches than recommended or wear them for longer than instructed. Both actions increase the total dose of lidocaine your body absorbs over time.
Handle and Dispose of Patches Correctly
You should wear nitrile gloves during application to avoid numbing your fingertips. Never apply a patch to the face or mucous membranes. Used patches still contain a significant amount of active drug, so they must be folded in half with the sticky sides together and disposed of carefully to prevent accidental exposure to children or pets.
Factors That Increase Systemic Risk
Several external and internal factors can increase the amount of lidocaine absorbed from a patch, raising the risk of systemic side effects.
The Impact of External Heat
Avoid using external heat sources like heating pads or electric blankets over the patch. Heat increases local blood flow, which can accelerate the rate of lidocaine absorption into your system.
Concurrent Use of Anesthetics
If you are using any other products that contain local anesthetic agents (e.g., creams, gels, sprays), you must inform your healthcare provider. The total amount of drug absorbed from all formulations must be considered together to prevent overdose.
Patient-Specific Vulnerabilities
Patients with severe hepatic (liver) impairment may metabolize lidocaine more slowly, leading to higher concentrations in the blood. Similarly, smaller individuals may be more susceptible to the effects of a standard dose.
Understanding the Risks and Side Effects
While generally safe when used as directed, lidocaine patches carry risks that require awareness and caution.
Local Site Reactions
The most common side effects are local skin reactions at the application site, such as irritation, redness, or a burning sensation. If this occurs, the patch should be removed and not reapplied until the irritation subsides.
Potential for Systemic Overdose
Overdose is a serious risk if a patch is cut, chewed, or ingested, or if too many patches are worn at once. Symptoms can be severe and require immediate medical attention.
Allergic Reactions
While rare, hypersensitivity to lidocaine or other components of the patch can occur. Patients with a known allergy to para-aminobenzoic acid (PABA) derivatives may have a cross-sensitivity, though this is not always the case.
Making the Right Choice for Your Goal
Your approach to using lidocaine patches should be guided by a clear understanding of your primary objective, always in consultation with a healthcare professional.
- If your primary focus is safe pain management: Prioritize applying the patch only to healthy, intact skin for the exact duration prescribed and be vigilant about proper disposal.
- If you use other medications or have liver conditions: Ensure your doctor knows about all other products you use (especially other anesthetics) and your full health history to prevent dangerous interactions or accumulation.
- If you are caring for a child or vulnerable adult: Be aware that overdose risk is higher; never use more patches than prescribed or apply an adult patch to a child.
Ultimately, informed and precise application is the key to leveraging the benefits of lidocaine patches while minimizing potential harm.
Summary Table:
| Key Consideration | Why It Matters |
|---|---|
| Application Time | Wearing a patch longer than prescribed increases systemic absorption and overdose risk. |
| Skin Integrity | Applying to broken or inflamed skin can dramatically increase the rate of lidocaine absorption. |
| External Heat | Heat sources (e.g., heating pads) increase blood flow, accelerating drug absorption. |
| Concurrent Anesthetic Use | Using other lidocaine products (creams, sprays) adds to the total systemic dose. |
| Patient Factors | Liver impairment or smaller body size can increase susceptibility to side effects. |
Need a reliable, precisely dosed lidocaine transdermal patch?
As a bulk manufacturer for healthcare distributors and brands, Enokon specializes in developing safe and effective transdermal patches. We understand that precise dosing and controlled release are paramount for patient safety.
Partner with us to benefit from:
- Custom R&D: Our technical expertise ensures your lidocaine patch formulation delivers the exact systemic exposure required for your target market.
- Quality & Reliability: We manufacture patches with consistent drug release profiles, helping you mitigate the risks associated with improper dosing.
- Scalable Production: From clinical trials to commercial supply, we provide high-volume manufacturing to meet your needs.
Let's develop a patch that prioritizes patient safety. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What systemic side effects can lidocaine patches cause? Minimizing Risks for Safe Pain Relief
- How is the lidocaine patch administered? A Step-by-Step Guide for Safe & Effective Pain Relief
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
















