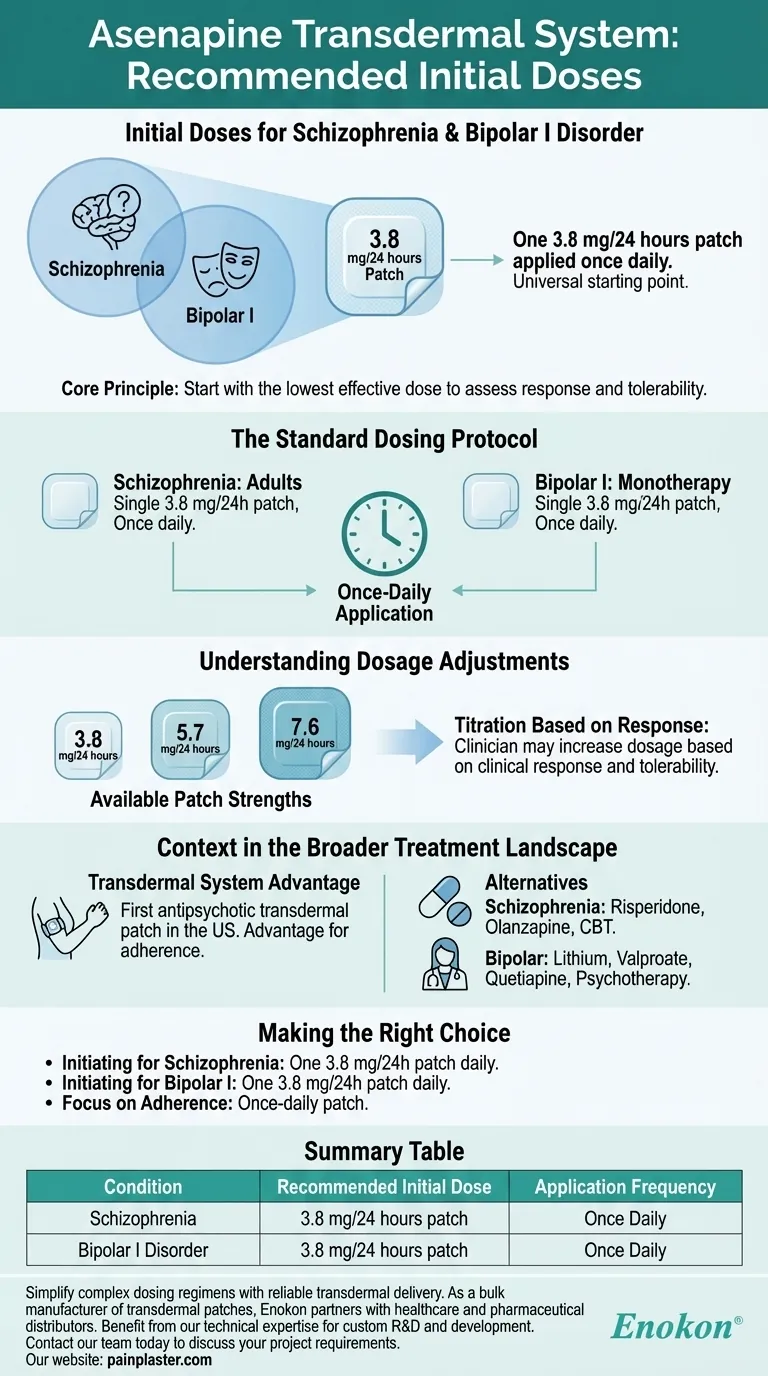
For both schizophrenia and bipolar I disorder, the recommended initial dose of the Asenapine transdermal system is one 3.8 mg/24 hours patch applied once daily. This starting dose is identical for both conditions and represents the lowest available strength.
The core principle of Asenapine transdermal system initiation is to begin with the lowest effective dose. The 3.8 mg/24 hours patch serves as a universal starting point for both schizophrenia and bipolar I, allowing clinicians to assess individual response and tolerability before considering any adjustments.
The Standard Dosing Protocol
Understanding the initial dosing is the first step in managing treatment with this unique delivery system. The protocol is designed for consistency and simplicity.
Initial Dose for Schizophrenia
For adults with schizophrenia, treatment begins with a single 3.8 mg/24 hours patch. This patch is applied to the skin once every 24 hours.
Initial Dose for Bipolar I Disorder
Similarly, for the monotherapy treatment of manic or mixed episodes associated with bipolar I disorder, the starting dose is one 3.8 mg/24 hours patch applied once daily.
A Single Daily Application
The transdermal system is designed for once-daily use. This simplifies the treatment regimen, which can be a significant factor in adherence.
Understanding Dosage Adjustments
The initial dose is not necessarily the final therapeutic dose. Treatment is a dynamic process tailored to the individual patient.
Available Patch Strengths
The Asenapine transdermal system is available in three strengths, providing a clear path for dose titration:
- 3.8 mg/24 hours
- 5.7 mg/24 hours
- 7.6 mg/24 hours
Titration Based on Response
A clinician may increase the dosage from the initial 3.8 mg/24 hours patch based on the patient's clinical response and how well they tolerate the medication. This flexibility allows for personalized care.
Why Start with the Lowest Dose?
Starting with the lowest available dose is a standard clinical practice. It helps minimize the risk of side effects while allowing the healthcare provider to carefully observe the medication's initial impact.
Context in the Broader Treatment Landscape
Asenapine is one of several options available, and its delivery method is a key differentiator.
The Significance of a Transdermal System
Asenapine holds a unique position as the first antipsychotic available in a transdermal patch formulation in the US. This can be a critical advantage for patients who have difficulty with oral medications.
Alternatives for Schizophrenia
The treatment landscape for schizophrenia is broad and includes other atypical antipsychotics like Risperidone and Olanzapine. Non-pharmacological approaches such as cognitive behavioral therapy (CBT) are also vital components of a comprehensive plan.
Alternatives for Bipolar Disorder
For bipolar disorder, treatment often involves mood stabilizers like Lithium or Valproate. Other atypical antipsychotics, such as Quetiapine, and various forms of psychotherapy are also common and effective options.
Making the Right Choice for Your Treatment Plan
The decision to use the Asenapine transdermal system should be made in consultation with a healthcare provider, considering the specific clinical situation.
- If your primary focus is initiating treatment for schizophrenia: The established starting point is one 3.8 mg/24 hours patch applied daily.
- If your primary focus is initiating treatment for bipolar I disorder: The protocol is identical, beginning with a single 3.8 mg/24 hours patch each day.
- If your primary concern is treatment adherence: A once-daily transdermal patch may offer a significant advantage over oral medication schedules.
Understanding the standard dosing protocol is a critical first step in making informed decisions about your therapeutic journey.
Summary Table:
| Condition | Recommended Initial Dose | Application Frequency |
|---|---|---|
| Schizophrenia | 3.8 mg/24 hours patch | Once Daily |
| Bipolar I Disorder | 3.8 mg/24 hours patch | Once Daily |
Simplify complex dosing regimens with reliable transdermal delivery. As a bulk manufacturer of transdermal patches, Enokon partners with healthcare and pharmaceutical distributors to bring treatments like Asenapine to market. Benefit from our technical expertise for custom R&D and development of your next-generation pain plasters and medicated patches. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health















