The transdermal oxybutynin system is a 39 cm² matrix-type patch containing 36 mg of racemic oxybutynin. It is engineered to deliver a consistent dose of 3.9 mg per day over a period of three to four days and is applied to the skin on the abdomen, hip, or buttock.
The key insight is that this patch isn't just an alternative to a pill; it's a fundamentally different delivery system designed to solve the problems of inconsistent drug levels and metabolic side effects that are common with oral medications.
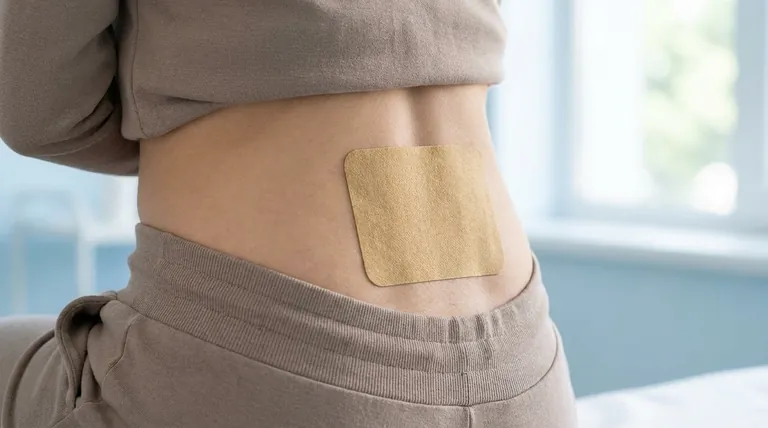
The Core Principle: Steady-State Delivery
A transdermal patch is designed to provide a continuous and controlled release of medication directly through the skin into the bloodstream. This approach offers a significant advantage over the fluctuating levels associated with taking pills.
Eliminating Peaks and Troughs
Oral medications often cause sharp spikes (peaks) in drug concentration shortly after being taken, followed by declines (troughs) before the next dose. The oxybutynin patch avoids this by releasing the drug at a slow, steady rate.
This consistency helps maintain a stable therapeutic level in the plasma, which can improve efficacy and reduce the side effects often linked to high peak concentrations.
Simplified Dosing and Compliance
The system is designed for a multi-day dosing interval, typically lasting three to four days.
Requiring only twice-weekly application dramatically improves patient compliance and convenience compared to remembering to take multiple oral doses every single day.
The Metabolic Advantage: Bypassing the Liver
One of the most significant benefits of transdermal delivery is its ability to bypass the digestive system and, more importantly, the liver's initial processing.
Avoiding First-Pass Metabolism
When a drug is taken orally, it passes through the liver before entering systemic circulation—a process known as first-pass hepatic metabolism. The liver breaks down a significant portion of the drug, which can reduce its effectiveness and create metabolites that cause side effects.
Direct-to-Bloodstream Efficacy
The transdermal patch delivers oxybutynin directly into the capillaries of the skin. This allows the drug to enter the bloodstream in its active form, avoiding the metabolic breakdown that occurs in the liver.
This direct route is a primary reason why transdermal systems often lead to a lower incidence of systemic side effects compared to their oral counterparts.
Understanding the Trade-offs
While transdermal delivery offers clear advantages, it also comes with inherent limitations that are important to understand. It is not a suitable method for all medications or all patients.
Constraint of Skin Permeability
The skin is a formidable barrier. Only certain types of drug molecules—typically those that are small and lipophilic (fat-soluble)—can effectively penetrate it.
The Need for Potent Medication
Because the rate of absorption through the skin is limited, transdermal delivery is only viable for drugs that are potent enough to be effective at very low doses. This method cannot be used for medications that require a large dose.
Potential for Skin Irritation
A common disadvantage is localized skin irritation or an allergic reaction at the application site. Patients must often rotate application sites to minimize this risk.
An Inherent Safety Feature
A unique advantage is the ability to immediately halt drug administration. If a patient experiences adverse effects, simply removing the patch stops the drug from entering the body.
Making the Right Choice for Your Goal
The decision to use a transdermal system over an oral medication depends entirely on the therapeutic goal and the patient's specific needs.
- If your primary focus is managing side effects: The transdermal system's ability to bypass the liver and maintain steady drug levels often results in a more tolerable experience.
- If your primary focus is convenience and adherence: A patch that only needs to be changed twice a week is significantly easier to manage than multiple daily pills.
- If you have sensitive skin or need a high dose of medication: The limitations of transdermal delivery, such as potential irritation and dosage constraints, may make an oral formulation a more suitable choice.
Ultimately, the transdermal oxybutynin system is a targeted therapeutic tool designed for efficacy and tolerability by fundamentally changing how the medication interacts with the body.
Summary Table:
| Specification | Details |
|---|---|
| Patch Size | 39 cm² matrix-type patch |
| Drug Load | 36 mg of racemic oxybutynin |
| Daily Dose | 3.9 mg per day |
| Wear Time | 3 to 4 days (twice-weekly application) |
| Key Benefit | Steady-state delivery, bypasses first-pass metabolism |
Ready to develop your own reliable transdermal patch?
As Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters, we partner with healthcare and pharma brands to bring their drug delivery concepts to life. Benefit from our technical expertise for custom R&D and development, ensuring a product that delivers consistent results for your patients.
Contact our experts today to discuss your project requirements.
Visual Guide

Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use














