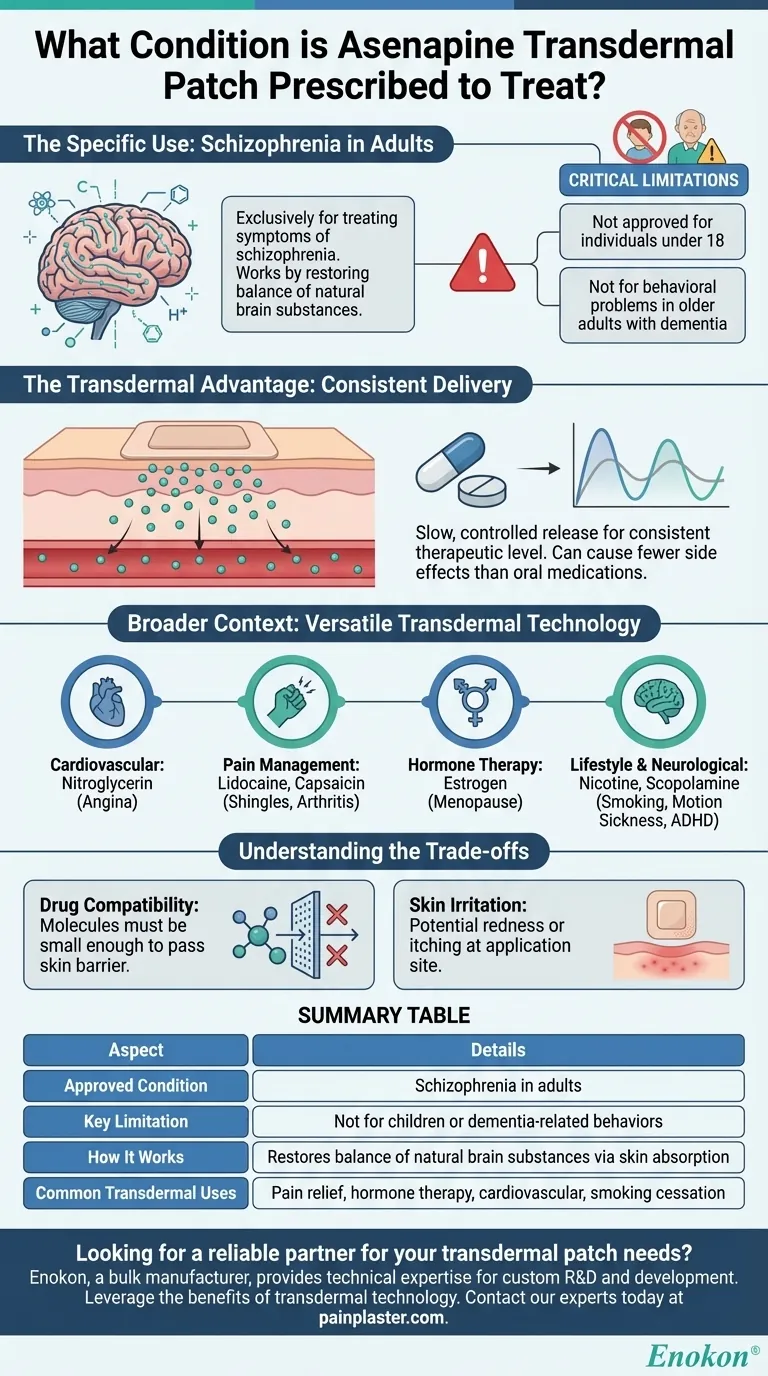
The asenapine transdermal patch is prescribed for a single, specific condition. It is used exclusively to treat the symptoms of schizophrenia in adults. Schizophrenia is a mental illness characterized by disturbed or unusual thinking, a loss of interest in life, and strong or inappropriate emotions. This medication is not approved for use in children and should not be used to treat behavioral problems in older adults with dementia.
While the asenapine patch is a highly specific treatment for schizophrenia, its delivery method—the transdermal patch—is a broad technology used to administer many different medications for a wide range of health issues.
The Specific Role of Asenapine
The effectiveness of this treatment comes from the combination of a specific drug (asenapine) with a specific delivery system (the transdermal patch).
Understanding Schizophrenia
Schizophrenia is a serious mental disorder that affects how a person thinks, feels, and behaves. The symptoms treated by asenapine often involve a disconnect from reality.
How Asenapine Helps
Asenapine is an antipsychotic medication that works by helping to restore the balance of certain natural substances in the brain. The goal is to manage the core symptoms of schizophrenia, leading to clearer thinking and more stable emotional states.
Critical Usage Limitations
It is crucial to note that this patch is only approved for adults. It has not been deemed safe or effective for individuals under 18 years of age.
Furthermore, a specific warning exists against its use for treating behavioral issues, such as agitation or aggression, in elderly patients who have dementia.
The Broader Context of Transdermal Delivery
The reason asenapine is delivered via a patch is rooted in the benefits of transdermal technology, which is used across many fields of medicine.
Why Use a Patch?
A transdermal patch delivers medication directly through the skin and into the bloodstream. This provides a slow, controlled release of the drug over a prolonged period.
This method ensures a consistent therapeutic level of medication in the body, which can be more effective and cause fewer side effects than the peaks and troughs associated with pills.
Examples Across Medicine
The technology behind the asenapine patch is highly versatile and proven. Other common uses for transdermal patches include:
- Cardiovascular Health: Nitroglycerin patches are used to prevent angina (chest pain) by improving blood flow to the heart.
- Pain Management: Lidocaine and capsaicin patches are applied directly to the skin to treat localized pain from conditions like shingles or arthritis.
- Hormone Therapy: Estrogen patches are commonly used to manage symptoms of menopause.
- Lifestyle & Neurological: Nicotine patches are a well-known tool for smoking cessation, and scopolamine patches are used to prevent motion sickness. Patches are also used for ADHD, Parkinson's disease, and depression.
Understanding the Trade-offs
While effective, the transdermal patch system is not a universal solution and comes with its own set of considerations.
Not All Drugs are Compatible
A medication can only be delivered via a patch if its molecules are small enough to pass through the skin barrier. This is why many common drugs are still only available in oral or injectable forms.
Potential for Skin Irritation
A common side effect of any transdermal patch is irritation, redness, or itching at the application site. This is a direct consequence of wearing an adhesive patch for an extended period.
Specificity is Non-Negotiable
Each transdermal patch is formulated for one specific drug and dose. A patch designed to deliver asenapine is fundamentally different from one that delivers nicotine or nitroglycerin and they can never be interchanged.
Making the Right Choice for Your Goal
Understanding the distinction between the drug and its delivery system is key to grasping its purpose.
- If you are prescribed an asenapine patch: Understand it is a targeted, long-term treatment for managing schizophrenia symptoms, designed to provide a steady and reliable dose of medication.
- If you are simply curious about the technology: Recognize that transdermal patches are a versatile medical tool used to consistently deliver a wide variety of medications for many different conditions.
Ultimately, knowing both the specific purpose of a medication and the reason for its delivery method empowers you to have more informed conversations about your healthcare.
Summary Table:
| Aspect | Details |
|---|---|
| Approved Condition | Schizophrenia in adults |
| Key Limitation | Not for use in children or for dementia-related behaviors |
| How It Works | Restores balance of natural brain substances via skin absorption |
| Common Transdermal Uses | Pain relief, hormone therapy, cardiovascular health, smoking cessation |
Looking for a reliable partner for your transdermal patch needs?
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise for custom R&D and development. Whether you are developing a new medication or optimizing an existing one, our team can help you leverage the benefits of transdermal technology.
Contact our experts today to discuss your project requirements and discover how we can support your success.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints















