Prescription capsaicin patches are primarily used to treat chronic neuropathic pain conditions, specifically postherpetic neuralgia (PHN) and diabetic neuropathy. These medicated patches contain 8% capsaicin, a compound derived from chili peppers, which works by temporarily desensitizing pain receptors in the skin. While over-the-counter capsaicin products may address milder pain, the prescription-strength patch is tailored for severe, persistent nerve pain resulting from shingles or diabetes complications. Its targeted application provides localized relief, making it a specialized tool in pain management protocols.
Key Points Explained:
-
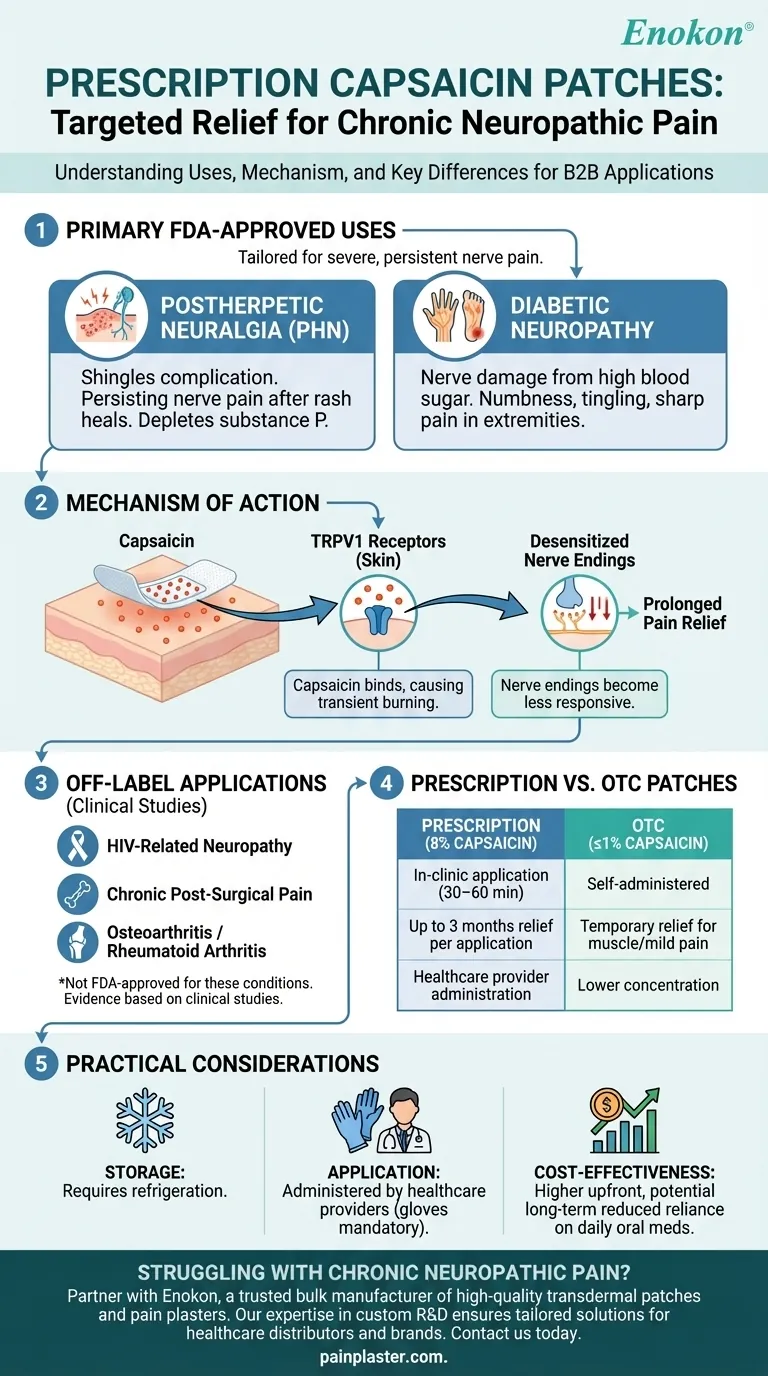
Primary FDA-Approved Uses
-
Postherpetic Neuralgia (PHN):
A complication of shingles (herpes zoster) where nerve pain persists for months or years after the rash heals. The patch alleviates burning, stabbing, or aching pain by depleting substance P, a neurotransmitter involved in pain signaling. -
Diabetic Neuropathy:
Nerve damage caused by high blood sugar levels, leading to numbness, tingling, or sharp pain in extremities (e.g., hands/feet). The 2020 FDA approval expanded its use for this condition, offering an alternative to oral medications.
-
Postherpetic Neuralgia (PHN):
-
Mechanism of Action
- Capsaicin binds to TRPV1 receptors in the skin, initially causing a burning sensation (transient side effect) followed by prolonged pain relief as nerve endings become less responsive.
- Unlike systemic treatments, the patch delivers high-concentration capsaicin directly to the affected area, minimizing systemic side effects.
-
Off-Label Applications
While not FDA-approved for these conditions, clinical studies suggest potential efficacy for:- HIV-Related Neuropathy
- Chronic Post-Surgical Pain
- Osteoarthritis/Rheumatoid Arthritis (though OTC patches are more common for joint pain).
-
Differentiation from OTC Patches
- Prescription patches (8% capsaicin) are applied in-clinic for 30–60 minutes, providing up to 3 months of relief per application.
- OTC patches (typically ≤1% capsaicin) are self-administered for temporary relief of muscle strains or mild arthritis pain.
-
Practical Considerations for Purchasers
- Storage: Requires refrigeration.
- Application: Must be administered by healthcare providers due to the risk of irritation (e.g., gloves are mandatory during handling).
- Cost-Effectiveness: Higher upfront cost but may reduce long-term expenses by decreasing reliance on daily oral medications.
For chronic pain patients unresponsive to conventional therapies, these patches represent a nuanced intersection of pharmacology and practical care—demonstrating how targeted treatments can transform quality of life.
Summary Table:
| Condition | Key Benefit | Application Notes |
|---|---|---|
| Postherpetic Neuralgia (PHN) | Alleviates persistent nerve pain after shingles by depleting pain-signaling substance P. | Applied in-clinic for 30–60 minutes. |
| Diabetic Neuropathy | Reduces numbness/tingling in extremities caused by nerve damage. | Requires healthcare provider administration. |
| Off-Label Uses* | Potential relief for HIV-related neuropathy or chronic post-surgical pain. | Evidence based on clinical studies. |
*Not FDA-approved for these conditions.
Struggling with chronic neuropathic pain? Partner with Enokon, a trusted bulk manufacturer of high-quality transdermal patches and pain plasters. Our expertise in custom R&D ensures tailored solutions for healthcare distributors and brands. Contact us today to discuss how our prescription-strength capsaicin patches can enhance your pain management offerings—backed by technical support and scalable production.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief















