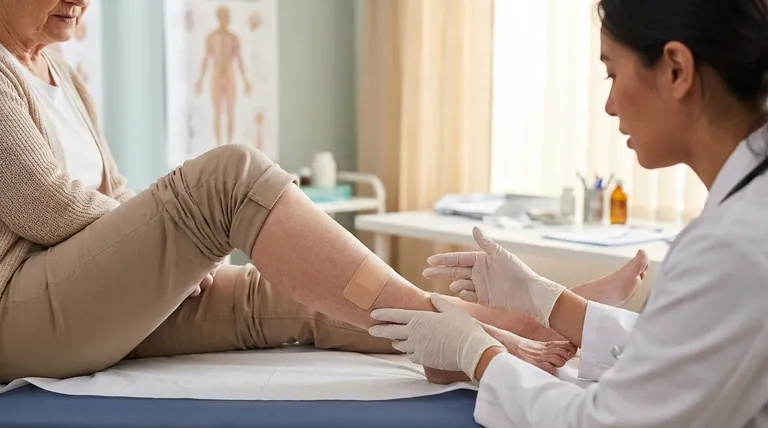
The prescription-strength capsaicin patch is specifically approved to treat two distinct and challenging types of chronic nerve pain. These are postherpetic neuralgia, the lingering pain that can follow a shingles infection, and painful diabetic neuropathy, a common complication causing nerve damage in the feet of people with diabetes.
The key distinction to understand is that the high-concentration (8%) prescription capsaicin patch is a targeted medical treatment for specific neuropathic (nerve-based) pain. It is fundamentally different from the lower-strength, over-the-counter versions used for general muscle and joint aches.
The Approved Conditions for Prescription Capsaicin
The regulatory approval for the prescription patch is narrow and focuses exclusively on pain originating from damaged nerves, not from muscle or joint inflammation.
Postherpetic Neuralgia (PHN)
Postherpetic neuralgia is a chronic pain condition that persists for months or even years after a shingles outbreak has cleared.
The pain is often described as a constant burning, stabbing, or aching sensation in the area where the shingles rash once was.
Painful Diabetic Neuropathy
This condition is a type of nerve damage that can occur as a complication of diabetes, most commonly affecting the feet and legs.
It manifests as numbness, tingling, and sharp, stabbing pains that can be severe and debilitating. The patch was approved for this use in 2020.
Why It's Prescribed for These Specific Conditions
The effectiveness of the prescription patch is directly related to its high concentration of capsaicin and its specific mechanism of action on nerve fibers.
A Targeted Attack on Pain Signals
Capsaicin works by targeting and over-stimulating the specific nerve endings responsible for sending pain signals to the brain.
This initial intense stimulation is followed by a prolonged period of desensitization, effectively "quieting" the dysfunctional nerves and providing significant pain relief. This mechanism is uniquely suited for neuropathic pain.
The Critical Role of High Concentration
The 8% concentration found in the prescription patch is necessary to achieve this nerve-desensitizing effect for severe, chronic conditions.
This potency is why its application must be handled in a clinical setting or with strict medical guidance.
Understanding the Trade-offs and Key Considerations
While effective, the prescription capsaicin patch is not a simple pain reliever. Its use requires careful medical oversight and a clear understanding of what it is—and is not—designed to do.
Prescription vs. Over-the-Counter (OTC)
The most common point of confusion is the difference between prescription and OTC patches.
OTC capsaicin products contain a much lower concentration and are intended for minor, temporary relief of muscle and joint pain, such as arthritis, backaches, and strains. They are not effective for severe neuropathic pain.
Critical Safety Protocols
The patch must only be applied to clean, dry, and intact skin. It should never be placed on skin that is cut, irritated, or damaged.
You must avoid applying external heat sources—such as heating pads, sunlamps, or hot tubs—over the patch area. You should also avoid showering or swimming immediately after application.
Medical Supervision is Non-Negotiable
Before using the patch, you must inform your doctor of any allergies, especially to salicylates (aspirin), menthol, or other topical products.
It is also vital to disclose your full medical history, including conditions like asthma or nasal polyps, and to inform all of your healthcare providers that you are using this treatment.
Making the Right Choice for Your Goal
Understanding the precise purpose of each type of capsaicin therapy ensures you are using the correct tool for your specific type of pain.
- If your primary focus is severe, lingering pain after a shingles infection: The prescription capsaicin patch is an approved, targeted therapy you should discuss with your doctor.
- If your primary focus is stabbing pain or numbness in the feet from diabetes: The prescription patch is also an approved medical treatment specifically for this type of neuropathic pain.
- If your primary focus is general muscle soreness, a minor backache, or joint stiffness: A lower-strength, over-the-counter capsaicin patch is the appropriate choice for this type of pain.
Ultimately, choosing the right treatment begins with an accurate diagnosis of the source of your pain.
Summary Table:
| Condition | Description | Key Patient Experience |
|---|---|---|
| Postherpetic Neuralgia (PHN) | Chronic nerve pain following a shingles outbreak. | Burning, stabbing, or aching pain in the area of the previous rash. |
| Painful Diabetic Neuropathy | Nerve damage in the feet/legs from diabetes. | Numbness, tingling, and sharp, stabbing pains. |
| Over-the-Counter (OTC) Patches | For general muscle and joint aches. | Arthritis, backaches, strains (not for neuropathic pain). |
Partner with Enokon for Your Transdermal Pain Relief Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for custom R&D and development. If you are looking to develop or source a high-quality, targeted pain relief patch, our team can help bring your product to market efficiently.
Contact our experts today to discuss your specific requirements and benefit from our manufacturing capabilities.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing













