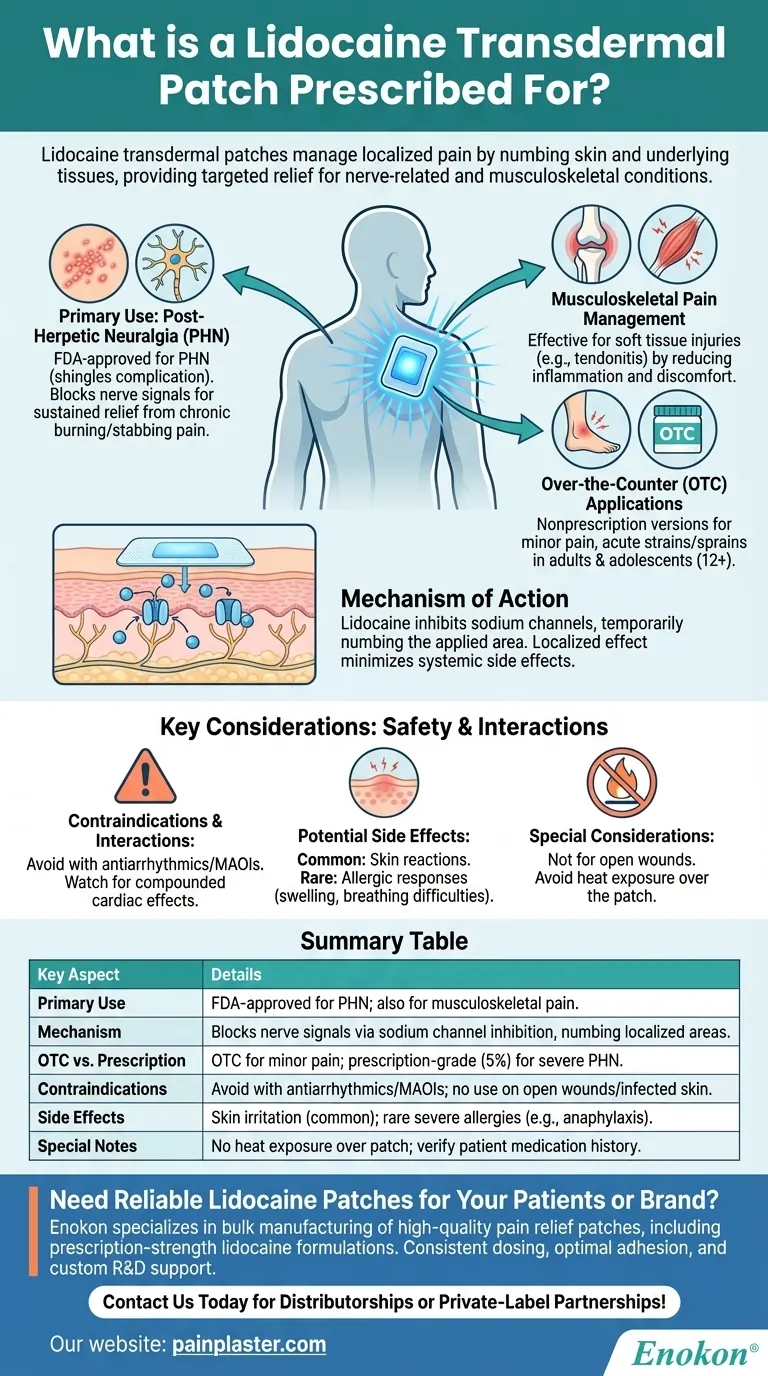
Lidocaine transdermal patches are primarily prescribed to manage localized pain, particularly nerve-related pain such as post-herpetic neuralgia (PHN), a persistent condition following shingles. They also address musculoskeletal pain from soft tissue injuries or tendonitis. The patches work by numbing the skin and underlying tissues, providing targeted relief. While prescription versions treat severe conditions like PHN, over-the-counter options are available for minor aches. However, they carry potential side effects and drug interactions, requiring careful use under medical supervision.

Key Points Explained:
-
Primary Use: Post-Herpetic Neuralgia (PHN)
- The Lidocaine Transdermal Patch is FDA-approved for PHN, a complication of shingles causing chronic burning or stabbing pain. It blocks nerve signals in the affected area, offering sustained relief where oral medications may fall short.
-
Musculoskeletal Pain Management
- Studies show efficacy for soft tissue injuries (e.g., tendonitis) by reducing inflammation and discomfort at the source. This makes it useful for athletes or individuals with repetitive strain injuries.
-
Over-the-Counter (OTC) Applications
- Nonprescription versions target minor pain in limbs, neck, or shoulders. Ideal for acute strains or sprains in adults and adolescents (12+ years), though potency is lower than prescription-grade patches.
-
Mechanism of Action
- Lidocaine inhibits sodium channels in nerve endings, temporarily numbing the applied area. This localized effect minimizes systemic side effects compared to oral painkillers.
-
Contraindications & Interactions
- Avoid with antiarrhythmics (e.g., amiodarone) or MAOIs due to risk of compounded cardiac effects. Concurrent use of other topical anesthetics (e.g., tetracaine) may increase toxicity.
-
Potential Side Effects
- Common: Skin reactions (redness, itching) at the application site.
- Rare but severe: Allergic responses like bronchospasm or anaphylaxis. Users should monitor for swelling or breathing difficulties.
-
Special Considerations
- Not for open wounds or infected skin. Patients should avoid heat exposure (e.g., heating pads) over the patch to prevent accelerated drug release.
-
Comparison to Other Patches
- Unlike hormone-delivering patches (e.g., estradiol for menopause), lidocaine patches are purely analgesic with no systemic hormonal effects.
For purchasers, key factors include prescription strength (5% lidocaine for PHN vs. lower OTC doses), patch size (matched to pain area), and adhesion quality for prolonged wear. Always verify patient medication history to prevent interactions.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Use | FDA-approved for post-herpetic neuralgia (PHN); also for musculoskeletal pain. |
| Mechanism | Blocks nerve signals via sodium channel inhibition, numbing localized areas. |
| OTC vs. Prescription | OTC for minor pain (e.g., sprains); prescription-grade (5%) for severe PHN. |
| Contraindications | Avoid with antiarrhythmics/MAOIs; no use on open wounds or infected skin. |
| Side Effects | Skin irritation (common); rare severe allergies (e.g., anaphylaxis). |
| Special Notes | No heat exposure over patch; verify patient medication history. |
Need reliable lidocaine transdermal patches for your patients or brand?
Enokon specializes in bulk manufacturing of high-quality pain relief patches, including prescription-strength lidocaine formulations. Our expertise in transdermal technology ensures consistent dosing, optimal adhesion, and custom R&D support for tailored solutions.
Contact us today to discuss your requirements for healthcare distributorships or private-label partnerships!
Visual Guide
Related Products
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How can you use lidocaine patches for multiple sore spots? A Guide to Safe, Effective Pain Relief
- When should someone contact a doctor regarding lidocaine patch use? Ensure Safe Pain Relief
- How should the treated area be protected while wearing a lidocaine patch? Safety Tips for Effective Pain Relief
- How are lidocaine patches typically used for pain relief during pregnancy? A Guide to Safe, Targeted Relief
- Is it safe to use lidocaine patches while breastfeeding? Expert Guidance for Nursing Mothers
















